Iron imido and nitrido complexes with highly oxidated iron centers are important in biocatalysis, for example, in ammonia synthesis. However, examples of such terminal Fe(V) or Fe(VI) imido and nitrido complexes obtained by chemical synthesis are rare. The existing examples are stabilized by bulky, chelating ligands, such as tripodal tris-phosphine or tris-N-heterocyclic carbene (NHC) ligands. Until now, there had only been one example of an iron(V) nitrido complex that was characterized by X-ray crystallography, i.e., a complex in which the Fe(V)≡N group is supported by a boron-based scorpionate-type tris-NHC ligand.
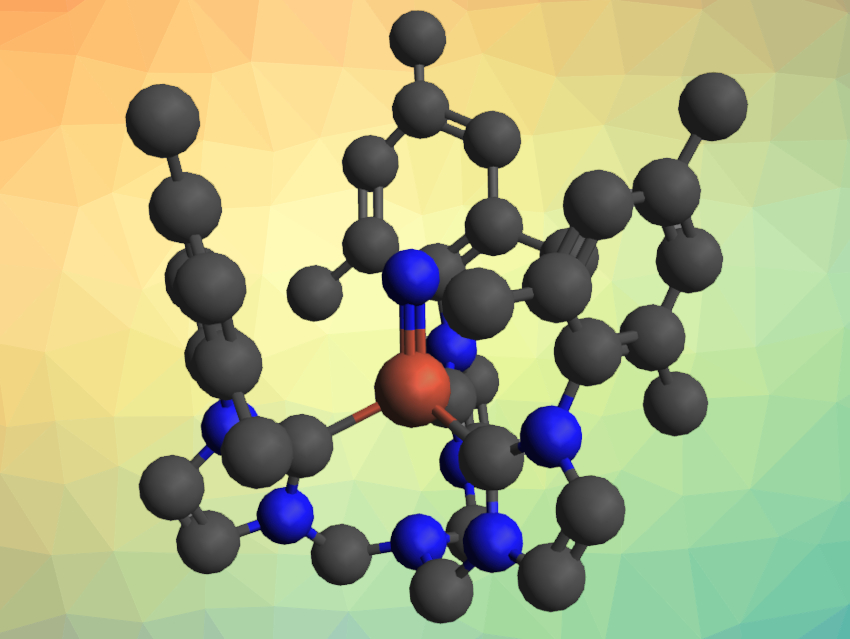
Karsten Meyer, Friedrich-Alexander-University Erlangen-Nürnberg, Erlangen, Germany, and colleagues have synthesized a new supporting ligand that allowed them to isolate an air-stable Fe(V) nitrido complex (pictured). The ligand, tris-[2-(3-mesityl-imidazol-2-ylidene)methyl]amine (TIMMNMes), has a central N atom that is connected to three NHC groups via CH2 bridges. The team used a TIMMNMes iron(II) chlorido complex as a starting material, which was first converted to an azide intermediate. This azide complex was then photolytically converted to the iron(IV) nitrido complex [(TIMMNMes)FeIVN](PF6) and finally oxidized with silver hexafluorophosphate to give the desired Fe(V) complex [(TIMMNMes)FeVN](PF6)2.
The product was characterized using Mössbauer and UV/Vis spectroscopy as well as single-crystal X-ray diffraction, which represents only the second X-ray diffraction analysis of an iron(V) nitrido complex so far. The team found that the oxidation from Fe(IV) to Fe(V) causes an elongation of the Fe≡N bond, while the iron moves closer to the plane defined by the binding C atoms of the NHC ligands, i.e., closer to the equatorial plane of a trigonal bipyramid. This change is enabled by the flexible chelate ligand. The Fe(V) complex is stable in the presence of air or water. According to the researchers, this could allow further detailed studies of this rare type of complex.
- Ligand Tailoring Toward an Air-Stable Iron(V) Nitrido Complex,
Martin Keilwerth, Liam Grunwald, Weiqing Mao, Frank W. Heinemann, Jörg Sutter, Eckhard Bill, Karsten Meyer,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.0c11141