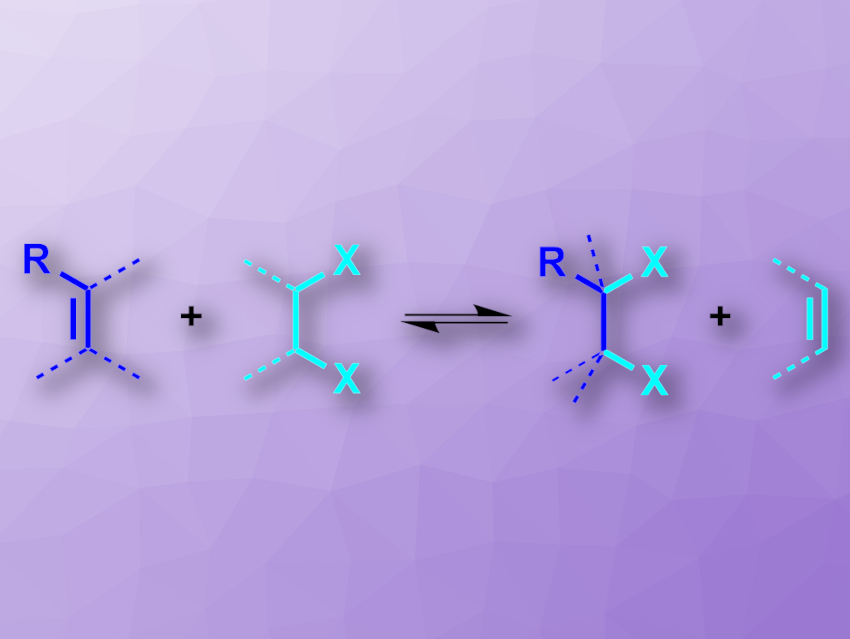
Some hydrogenation reactions can be performed using a liquid hydrogen source, e.g., an alcohol, instead of elemental H2—a so-called transfer hydrogenation. Liquid reactants are generally easier to handle than gaseous ones. This concept could be particularly useful for reactions that are usually performed using Cl2 or Br2, which are hard to handle and toxic. Vicinal dibromides and dichlorides, for example, can be prepared by dihalogenations of alkenes. However, catalytic, reversible transfer functionalization processes of alkenes have so far been limited to mono-/hydrofunctionalizations—i.e., they can only introduce one halogen atom at a double bond, not two.
Siegfried R. Waldvogel, Johannes Gutenberg University, Mainz, Germany, Bill Morandi, Swiss Federal Institute of Technology (ETH) Zurich, Switzerland, and colleagues have developed an electrochemically assisted “e-shuttle” process for the interconversion of alkenes and vicinal dihalides (pictured). The team used 1,2-dibromoethane, 1,2-dichloroethane, or 1,1,1,2-tetrachloroethane as reagents to synthesize dihalogenated molecules from alkenes in an electrochemical setup with graphite electrodes, Et4NBF4 as an electrolyte, 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) as an additive, and acetonitrile as the solvent. The desired dihalogenated products were obtained in moderate to good yields.
The “e-shuttle” reaction is reversible, which makes it possible to also use it for retro-dihalogenation reactions, i.e., for removing halogens. This can be useful to degrade halogenated compounds, which can be environmentally harmful. The researchers demonstrated this principle by directly using soil contaminated with lindane (gamma-hexachlorocyclohexane) in the reaction. This approach could reduce pollution and give useful chlorinated products at the same time.
- Merging shuttle reactions and paired electrolysis for reversible vicinal dihalogenations
Xichang Dong, Johannes L. Roeckl, Siegfried R. Waldvogel, Bill Morandi,
Science 2021.
https://doi.org/10.1126/science.abf2974