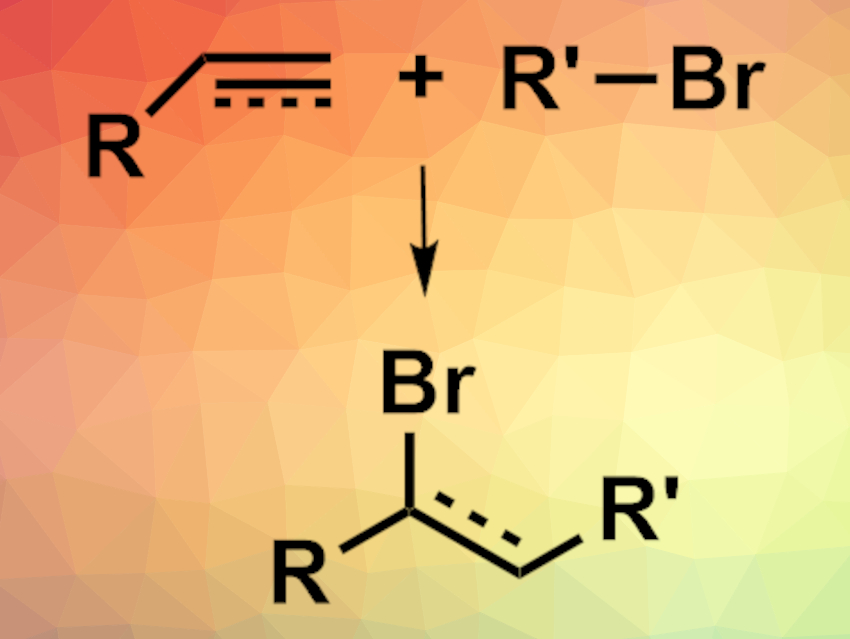Photocatalyzed organic reactions can often be used to achieve transformations under mild conditions. However, such processes generally use photocatalysts based on rare transition metals such as ruthenium or iridium. Bi2O3 could be a greener, non-toxic, low-cost alternative catalyst. However, a phase change during the reaction suggests that a soluble bismuth species, not the solid Bi2O3, is the catalytically active species in organic reactions that use Bi2O3 as a photocatalyst. Identifying this species could help to understand the catalytic process and improve its efficiency.
Paola Riente, Timothy Noël, Eindhoven University of Technology, The Netherlands, and colleagues have investigated the active photocatalytic species in a model atom transfer radical addition (ATRA) reaction using Bi2O3 (pictured). The team used an addition reaction between diethyl bromomalonate (DEBM) and 5-hexen-1-ol as the model. During the reaction, the mixture changes from a suspension to a yellowish solution, which indicates a soluble catalytically active species.
The researchers combined theoretical and experimental studies to identify this species. They performed UV–Vis spectroscopy, kinetic experiments, and density functional theory (DFT) calculations. They conclude that the “heterogeneous” photocatalysis with Bi2O3 in the presence of alkyl bromides actually involves a soluble BinBrm species. These compounds are closely related to pure BiBr3 or BiBr3-based solvent complexes. However, BiBr3 itself is highly hygroscopic, which makes Bi2O3 a useful, easier-to-handle solid precatalyst.
- Shedding light on the nature of the catalytically active species in photocatalytic reactions using Bi2O3 semiconductor,
Paola Riente, Mauro Fianchini, Patricia Llanes, Miquel A. Pericàs, Timothy Noël,
Nat. Commun. 2021.
https://doi.org/10.1038/s41467-020-20882-x