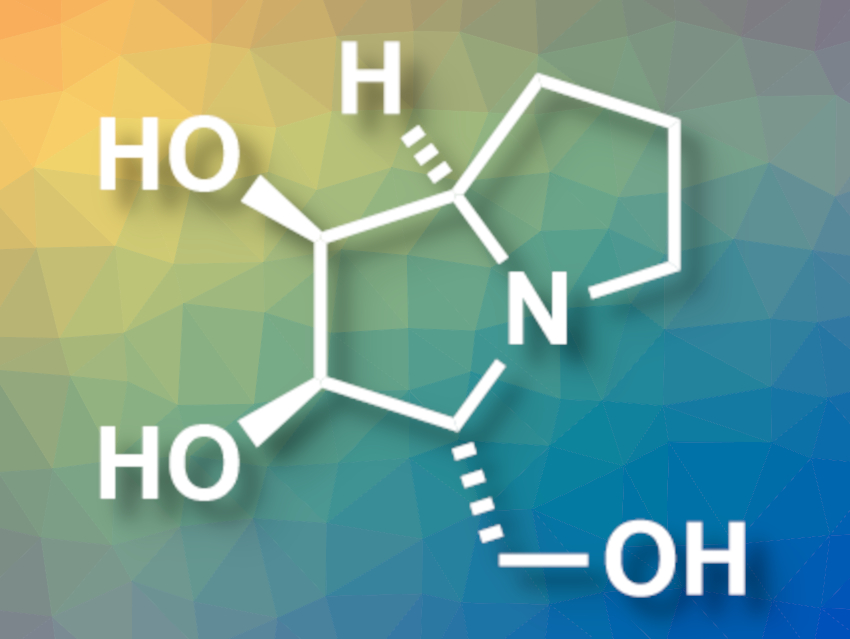
(+)-Hyacinthacine A1 (pictured) is a pyrrolizidine derivative that acts as a glycosidase inhibitor at a low micromolar range. This makes it interesting for biomedical studies. However, (+)-hyacinthacine A1 is challenging to synthesize.
Michel Gravel, University of Saskatchewan, Saskatoon, Canada, and colleagues have developed the shortest synthesis of (+)-hyacinthacine A1 to date. The team started from commercially available D-serine methyl ester, which was converted to an amino aldehyde derivative with a doubly protected amine. This intermediate was used in an aldehyde–aldehyde cross-benzoin reaction with furfural, i.e., an aldehyde-substituted furan. This reaction gave an α-hydroxy-β-aminoketone. This ketone was reduced to the corresponding alcohol and both protecting groups at the amine were removed to give a free aminodiol.
A complex photooxygenation–amine cyclization cascade, followed by hydrogenation and hydrogenolysis, then gave the desired product as a single diastereomer with an overall one-pot process yield of 27 % from the aminodiol. According to the researchers, the method might also be suitable for other diastereomers or similar synthetic targets.
- Total Synthesis of (+)-Hyacinthacine A1 Using a Chemoselective Cross-Benzoin Reaction and a Furan Photooxygenation–Amine Cyclization Strategy,
Karnjit Parmar, Pouyan Haghshenas, Michel Gravel,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c00090