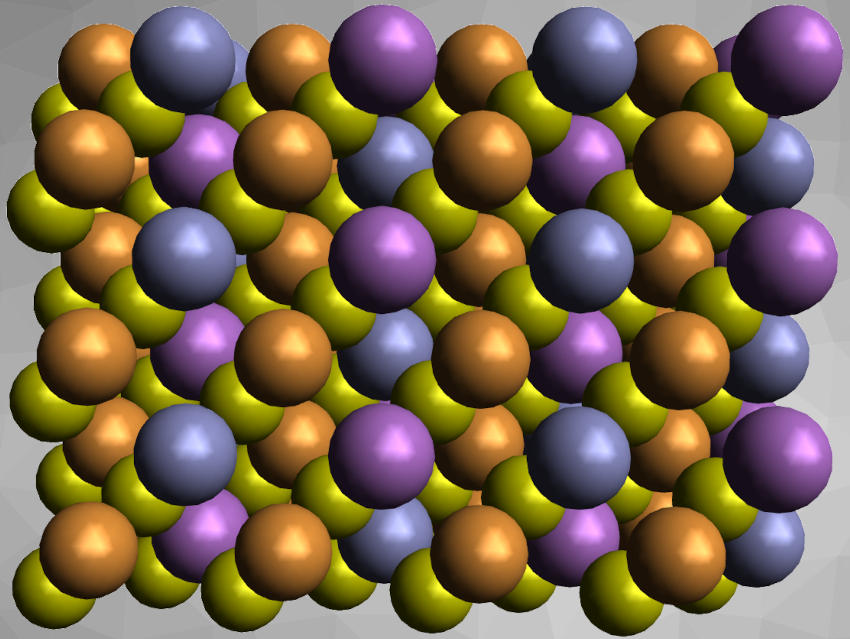
Compounds of the type A2IBIICIVX4 constitute a well-known family of materials with a large number of different members (A = Cu,Ag,Li,Na; B = Mn,Fe,Co,Ni,Zn,Cd,Hg,Mg,Ca,Sr,Ba,Pb,Eu; C = Si,Ge,Sn; X = S,Se,Te). These compounds have applications as tunable semiconductors, e.g., in solar cell absorbers or as photocatalysts for water splitting. In compounds with A = Cu, copper usually has the oxidation state +I. Replacing the CIV component in such copper compounds with a trivalent cation, such as Sb3+, might result in mixed-valent Cu+/Cu2+ species.
Martin Lerch, Technical University of Berlin, Germany, and colleagues have investigated this type of replacement and substituted the tin in Cu2ZnSnS4 with antimony. They synthesized a new member of the A2IBIICIVX4 family, Cu2ZnSbS4. The team prepared this compound by ball milling the corresponding binary sulfides, i.e., CuS, ZnS, Sb2S3, as well as Cu together. After milling, the samples were annealed at 250 °C in an H2S atmosphere.
The product was characterized by X-ray powder diffraction. The team found that Cu2ZnSbS4 crystallizes in a stannite-type structure in space group I42m. The team measured the magnetic properties of the compound to check for mixed-valent copper, but found no evidence for the presence of Cu2+ or Sb3+. Instead, theoretical calculations and the results of 121Sb Mössbauer spectroscopy indicate remarkably strong covalent SbV–S and Sb–Sb bonding.
- Cu2ZnSbS4: A Thioantimonate(V) with Remarkably Strong Covalent Sb–S Bonding,
Eva M. Heppke, Steffen Klenner, Oliver Janka, Rainer Pöttgen, Thomas Bredow, Martin Lerch,
Inorg. Chem. 2021.
https://doi.org/10.1021/acs.inorgchem.0c03601