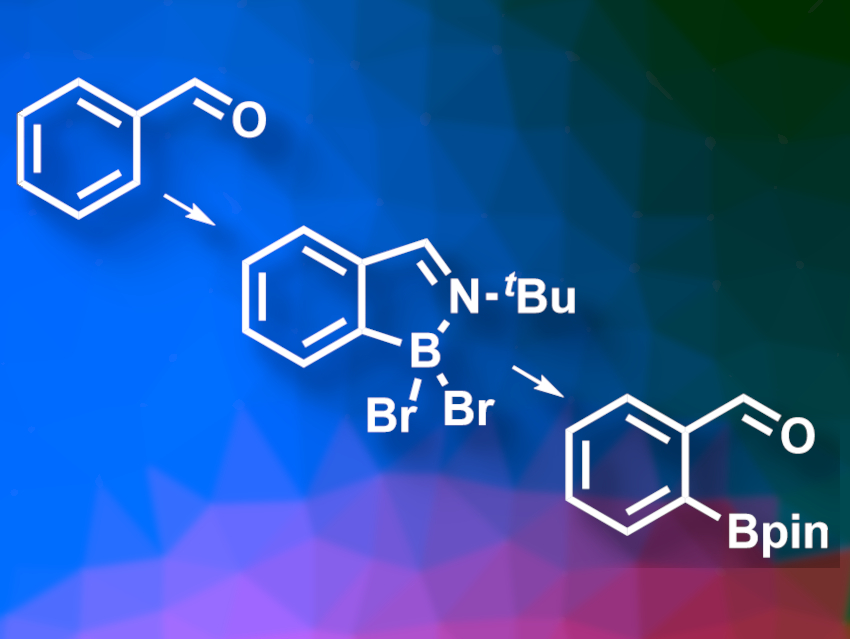
Transition-metal-catalyzed C–H functionalizations are commonly used in organic synthesis. C–H borylations, for example, can be employed to prepare useful organoboron intermediates. Directing groups can be used to perform such borylations in a regioselective manner. However, this is usually not step-economical because the directing group needs to be installed before and removed after the borylation. Transient directing groups can help with this issue: Their installation and removal can be performed in a one-pot reaction. Developing metal-free reactions using transient directing groups would have additional advantages, e.g., lower costs and the avoidance of metal residues in the final products.
Supriya Rej and Naoto Chatani, Osaka University, Japan, have developed an approach for the selective C–H borylation of electron-deficient benzaldehyde derivatives via a metal-free reaction using a transient imine directing group (pictured). The team converted a variety of substituted benzaldehydes to the corresponding ortho-functionalized pinacol boronates. First, the aldehydes were reacted with tert-butylamine to convert them to imines and then with BBr3 to form an electrophilic boron species. A reaction with pinacol in the presence of NEt3 then gave the desired pinacol boronates.
The products were obtained in good to excellent yields, with good ortho-selectivity even in the presence of other potential directing groups. The obtained pinacol boronates can be used in further functionalization reactions.
- Transient Imine as a Directing Group for the Metal-Free o-C–H Borylation of Benzaldehydes,
Supriya Rej, Naoto Chatani,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.0c13013