Samarium(II) iodide (SmI2), or Kagan’s reagent, is a strong one-electron reducing agent often used in organic chemistry. It is typically used in superstoichiometric amounts. Using SmI2 in a catalytic manner could potentially reduce both waste and costs. However, existing examples of SmI2-catalyzed reactions require the use of superstoichiometric amounts of a metal co-reductant to regenerate the Sm(II) catalyst.
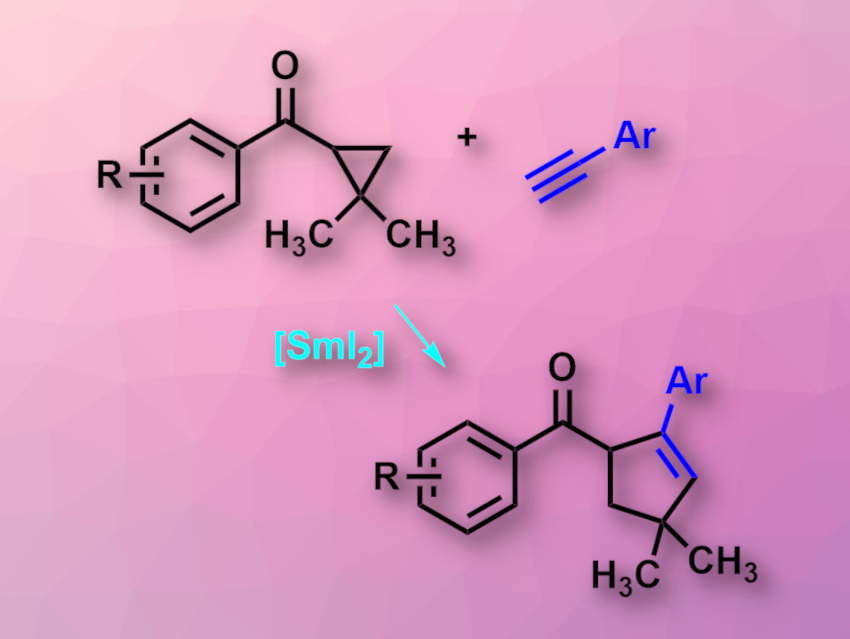
David J. Procter, The University of Manchester, UK, and colleagues have developed an SmI2-catalyzed intermolecular radical coupling of aryl cyclopropyl ketones and alkynes to give cyclopentenes (pictured). The reaction uses loadings of SmI2 as low as 15 mol% and does not require a superstoichiometric co-reductant to regenerate SmI2. The team used a range of cyclopropyl phenyl ketones and a wide variety of aryl alkynes as coupling partners, 15–25 mol% of SmI2 as the catalyst, and tetrahydrofuran (THF) as the solvent. The reaction was performed at 45 °C.
The desired cyclopentene products were obtained in good to excellent yields. The reaction tolerates some functional groups that are typically reduced by non-catalytic amounts of SmI2. The team proposes a radical-relay mechanism for the intermolecular coupling, starting with a reversible single-electron transfer from SmI2 to the ketone, which then leads to ring-opening of the cyclopropyl group. Intermolecular coupling with the alkyne and closing of the cyclopentene ring then gives a ketyl radical. This radical can transfer an electron back to Sm(III), regenerate the catalyst, and liberate the product.
- SmI2-Catalyzed Intermolecular Coupling of Cyclopropyl Ketones and Alkynes: A Link between Ketone Conformation and Reactivity,
Soumitra Agasti, Nicholas A. Beattie, Joseph J. W. McDouall, David J. Procter,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c01356