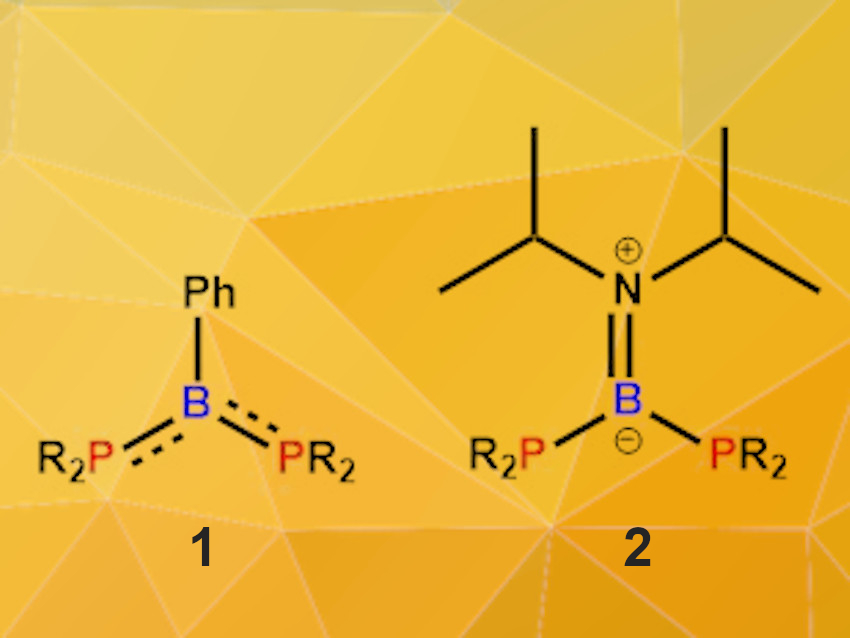
The activation of small molecules is an important and often challenging field of chemistry. Many of these reactions depend on expensive and toxic transition-metal catalysts. Compounds of abundant main group elements, such as phosphorus and boron, in low oxidation states could serve as useful alternatives to these catalysts. Diphosphinoboranes are examples of this type of compound. They can be seen as intramolecular frustrated Lewis pairs with two Lewis-basic P centers and one Lewis-acidic B center in a single molecule.
Rafał Grubba, Gdańsk University of Technology, Poland, and colleagues have found that P–B–P systems can activate small molecules. The team investigated the reactivity of two diphosphinoborane model compounds, i.e., (tBu2P)2BPh (1, pictured on the left, R = tBu) and (Cy2P)2BNiPr2 (2, pictured on the right, R = Cy). In 1, the lone pairs at phosphorus interact with the Lewis-acidic boron center, forming P–B-bonds with partial double-bond character. In 2, the amino group has a strong donor ability, leading to long P–B single bonds. This changes the nucleophilic/electrophilic properties of the P and B reactive centers: 1 has an electrophilic boron center and nucleophilic P atoms, while in 2, the electron-donating amino group significantly reduces the electrophilic properties of the boron atom and only the nucleophilic P centers remain.
Compound 1 can react with hydrogen to give tBu2PH and a dimeric hydrogen addition product, or with CO2 to form a product in which CO2 has inserted into a B–P-bond of the diphosphinoborane. Compound 2 does not react with H2, but forms a CO2 insertion product. Both diphosphinoboranes can also react with phenyl isocyanate.
The presence of a nucleophilic boron center is crucial for the activation of H2, whereas nucleophilic phosphorus centers are necessary for the activation of CO2 and isocyanate. Tuning these properties by modification of the substituents at the respective atoms allows for the design of specific systems suitable for certain activation reactions.
- Diphosphinoboranes as Intramolecular Frustrated Lewis Pairs: P–B–P Bond Systems for the Activation of Dihydrogen, Carbon Dioxide, and Phenyl Isocyanate,
Natalia Szynkiewicz, Anna Ordyszewska, Jarosław Chojnacki, Rafał Grubba,
Inorg. Chem. 2021.
https://doi.org/10.1021/acs.inorgchem.0c03563