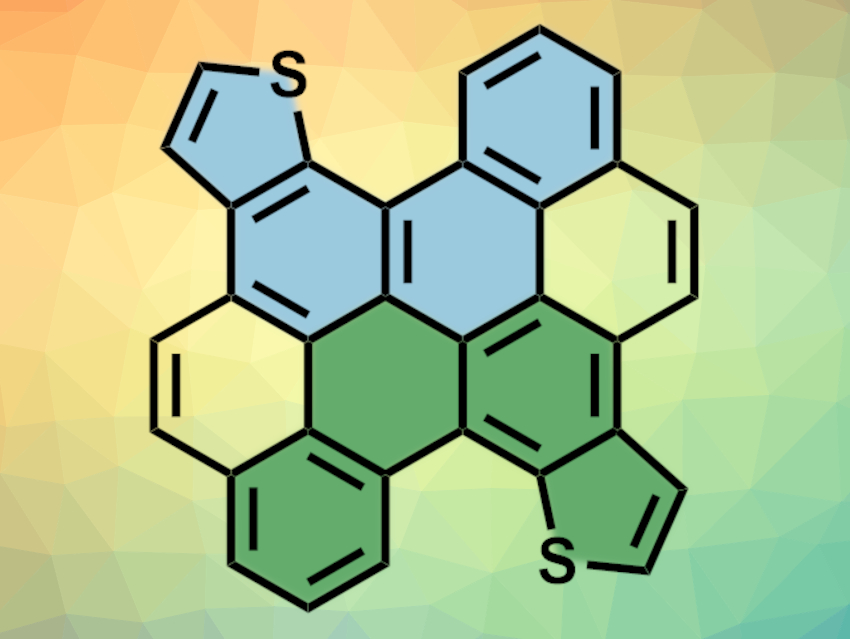
Nanographenes can be considered large polycyclic aromatic hydrocarbons (PAHs)—or small pieces of graphene nanoribbons. They could be useful materials with interesting electronic and optical properties. Graphene-like structures are usually planar. However, curved structures in nanographenes can be introduced, e.g., using strain from helicene-like substructures.
Yiyong Mai, Shanghai Jiao Tong University, China, Junzhi Liu, The University of Hong Kong, China, and colleagues have prepared three new sulfur-containing nanographenes that feature multiple subhelicenes (example pictured, helicene substructures in blue and green). The team first synthesized 4,4′-dibromo-2,2′-didodecyl-10,10′-biphenanthro[2,1-b]thiophene, which was then used to create precursors for the desired nanographenes. The team removed the two bromo-substituents to give a precursor for a nanographene containing two helicene substructures, or they introduced substituents with more aromatic rings to obtain precursors for larger nanographenes with four or six helicene substructures.
The desired products were then obtained via ring-closing reactions. They feature different types of helicene units, i.e., carbo[4]helicenes, thieno[4]helicenes, carbo[5]helicenes, and thieno[5]helicenes. The helicenes are non-planar due to steric interactions, with dihedral angles of 15°–34° as predicted by density functional theory (DFT) calculations. They could be useful, e.g., in optoelectronic devices.
- Sulfur-Doped Nanographenes Containing Multiple Subhelicenes,
Wenhui Niu, Yubin Fu, Hartmut Komber, Ji Ma, Xinliang Feng, Yiyong Mai, Junzhi Liu,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c00232