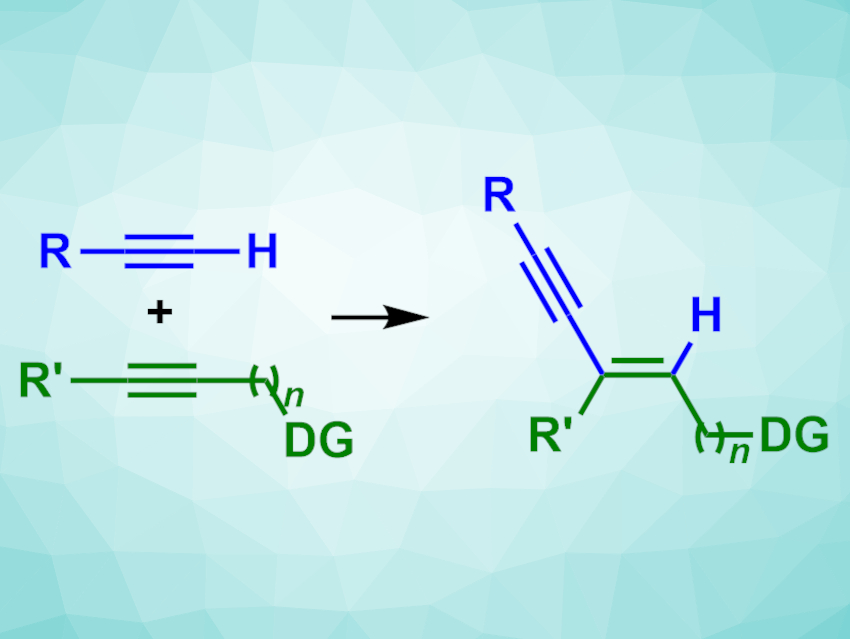
C–C cross-coupling reactions are often used in organic synthesis. However, they usually require prefunctionalized reactants, which leads to a higher number of reaction steps and reduces efficiency. A high efficiency is particularly important in large-scale reactions, such as in the chemical and pharmaceutical industry.
Keary M. Engle, The Scripps Research Institute, La Jolla, CA, USA, and colleagues have developed a selective, catalytic method for the cross-coupling of internal and terminal alkynes to give 1,3-enynes that does not require prefunctionalized building blocks. The team used internal alkynes with a donor group (DG)—such as alcohols, amines, or amides—and different terminal alkynes as coupling partners, Pd(dba)2 (dba = dibenzylideneacetone) as the catalyst together with a phosphinoimidazoline ligand, and ammonium acetate as an additive.
The desired 1,3-enynes were obtained in generally good yields and with high regio-and stereoselectivities. To demonstrate the scalability of the reaction, the team performed a model reaction with a propargyl alcohol on a gram scale, obtaining a yield of 94 %.
- Atom-Economical Cross-Coupling of Internal and Terminal Alkynes to Access 1,3-Enynes,
Mingyu Liu, Tianhua Tang, Omar Apolinar, Rei Matsuura, Carl A. Busacca, Bo Qu, Daniel R. Fandrick, Olga V. Zatolochnaya, Chris H. Senanayake, Jinhua J. Song, Keary M. Engle,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.0c12565