Converting the greenhouse gas CO2 into useful chemicals, such as fuels, could be an important aspect of a sustainable economy. Achieving such reactions under ambient conditions and with the CO2 drawn directly from air can be challenging, and usually, gaseous hydrogen and an external energy input are needed.
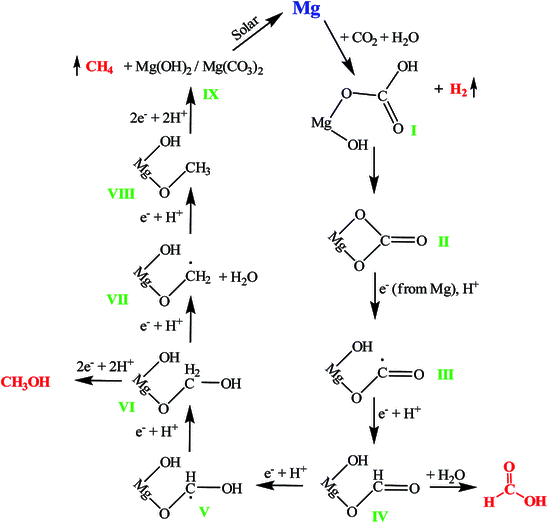
Vivek Polshettiwar, Tata Institute of Fundamental Research (TIFR), Mumbai, India, and colleagues have developed a reaction of magnesium with CO2 at room temperature and atmospheric pressure to give methane, methanol, and formic acid without requiring external energy sources and using water as the only hydrogen source. The team used magnesium nanoparticles—or even bulk magnesium—and reacted it with either pure CO2 or CO2 from air in water. Mg nanoparticles provided a higher reaction rate than bulk powders or granules due to the increased surface area.
The team found that the magnesium nanoparticles produce methane in amounts of about 100 μmol/g within 15 min, as well as 25.6 μmol/g of methanol and 26.2 μmol/g of formic acid, with a selectivity of 65.8 %, 16.7 %, and 17.2 % for methane, methanol, and formic acid, respectively. The efficiency of the conversion is, in some respects, better than that of known catalytic processes, and it does not require an external light or heat source. While the overall process is non-catalytic, magnesium is inexpensive and abundant. In addition, regenerating Mg(II) to obtain magnesium metal does not require large amounts of energy. The researchers propose that such a CO2 conversion reaction could be useful in the future, e.g., on the surface of Mars, where all necessary reagents have been found to be present.
- Direct CO2 capture and conversion to fuels on magnesium nanoparticles under ambient conditions simply using water,
Sushma A. Rawool, Rajesh Belgamwar, Rajkumar Jana, Ayan Maity, Ankit Bhumla, Nevzat Yigit, Ayan Datta, Günther Rupprechter, Vivek Polshettiwar,
Chem. Sci. 2021.
https://doi.org/10.1039/d1sc01113h