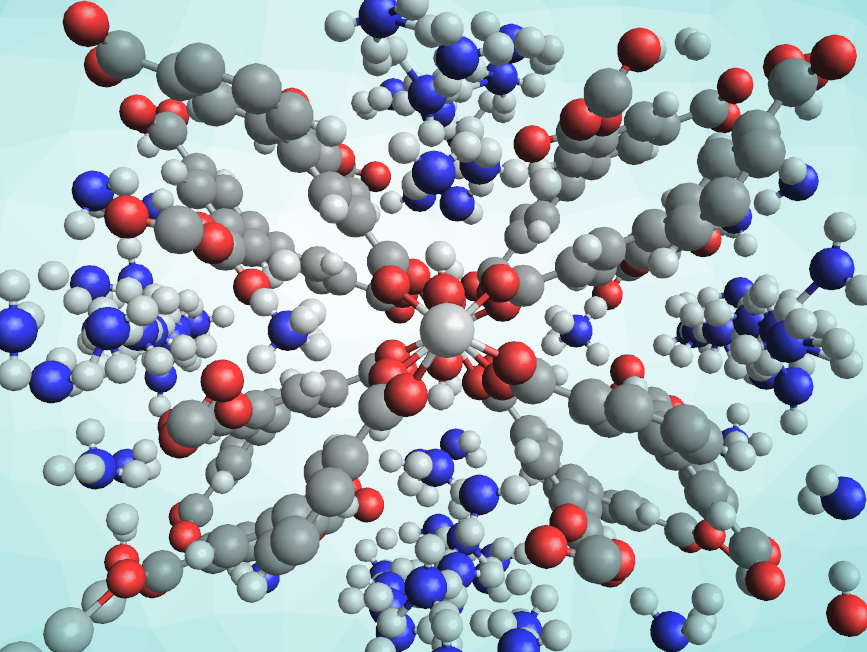
Metal–organic frameworks (MOFs) are porous materials composed of organic ligands and metal centers. They have applications, e.g., in gas storage and separation or sensing. Storing ammonia in MOFs can be challenging due to its reactive nature. MOFs used for this purpose need to be robust to remain stable over multiple adsorption–desorption cycles. In addition, some of the existing NH3 storage materials have issues such as a low ammonia packing density/a low storage capacity. Acidic sites could improve the adsorption of NH3 in porous materials.
Sihai Yang, Martin Schröder, University of Manchester, UK, and colleagues have developed a robust aluminium-based metal–organic framework (pictured), called MFM-303(Al) and functionalized with free carboxylic acid and hydroxyl groups, for the reversible adsorption of ammonia. The team prepared the MOF from biphenyl-3,3′,5,5′-tetracarboxylic acid and AlCl3 under acidic conditions. X-ray diffraction data showed that in MFM-303(Al), each Al(III) center is coordinated octahedrally to four oxygen atoms from carboxylate groups and two from bridging hydroxyl groups. Two carboxylate groups of each ligand remain free.
The MOF’s structure allows for a very high NH3 packing density of 0.801 g cm–3 at 293 K. For comparison, solid NH3 has a packing density of 0.817 g cm–3 at 193 K. The adsorption of ammonia in MFM-303(Al) is reversible. Even for wet NH3, only a small reduction of ca. 20 % in the uptake capacity was observed, and the MOF remained stable. According to the researchers, the efficient packing of ammonia in the framework is due to the effects of free carboxylic and hydroxyl groups.
- Exceptional Packing Density of Ammonia in a Dual-Functionalized Metal–Organic Framework,
Christopher Marsh, Xue Han, Jiangnan Li, Zhenzhong Lu, Stephen P. Argent, Ivan da Silva, Yongqiang Cheng, Luke L. Daemen, Anibal J. Ramirez-Cuesta, Stephen P. Thompson, Alexander J. Blake, Sihai Yang, Martin Schröder,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c01749