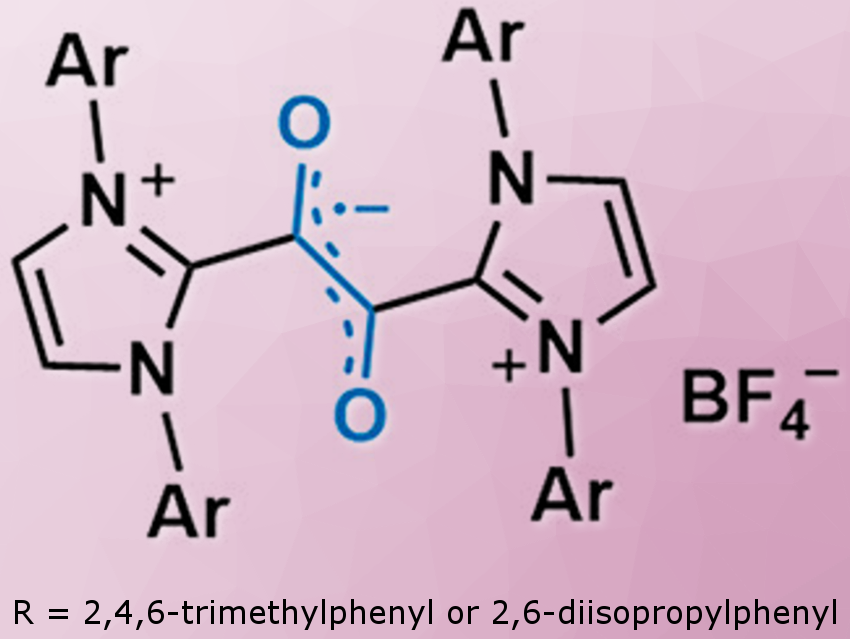
Air- and water-stable organic radicals are promising, for example, for the development of gadolinium-free organic radical contrast agents for magnetic resonance imaging (MRI). Apart from triphenylmethyl and TEMPO derivatives, air- and water-stable organic radicals are rare; their development remains a challenge.
Eunsung Lee and colleagues, Pohang University of Science and Technology (POSTECH), Republic of Korea, have synthesized organic radicals based on a 1,2-dicarbonyl framework supported by N-heterocyclic carbenes (NHCs; pictured). X-ray crystallography, EPR spectroscopy, and DFT calculations indicate that the unpaired electron is located on the central C2O2 unit.
The radical cations showed remarkable stability toward various chemical and physiological conditions: For example, they showed high resistance to various radical quenchers including peroxide, thiol, and ascorbate, but also remained stable in the presence of excess sodium hydroxide and under biological conditions (KP buffer and blood serum). The researchers measured a half-life of ∼2 h even in 0.1 M sulfuric acid. The thermal decomposition temperature is above 300 °C. These values are among the highest reported to date for organic radicals. Cyclic voltammetry showed that the radical cations are stable in the potential window between −1 and +0.5 V against saturated Ag/AgCl electrode.
The radical cations have no π-delocalization over the NHC fragments, which is quite common for NHC-derived stable radicals. The researchers attribute the successful design of the highly stable radicals to the steric and electronic stabilization provided by the two NHC moieties.
- Highly Stable 1,2-Dicarbonyl Radical Cations Derived from N-Heterocyclic Carbenes,
Youngsuk Kim, Jung Eun Byeon, Gu Yoon Jeong, Seoung Su Kim, Hayoung Song, Eunsung Lee,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c00707