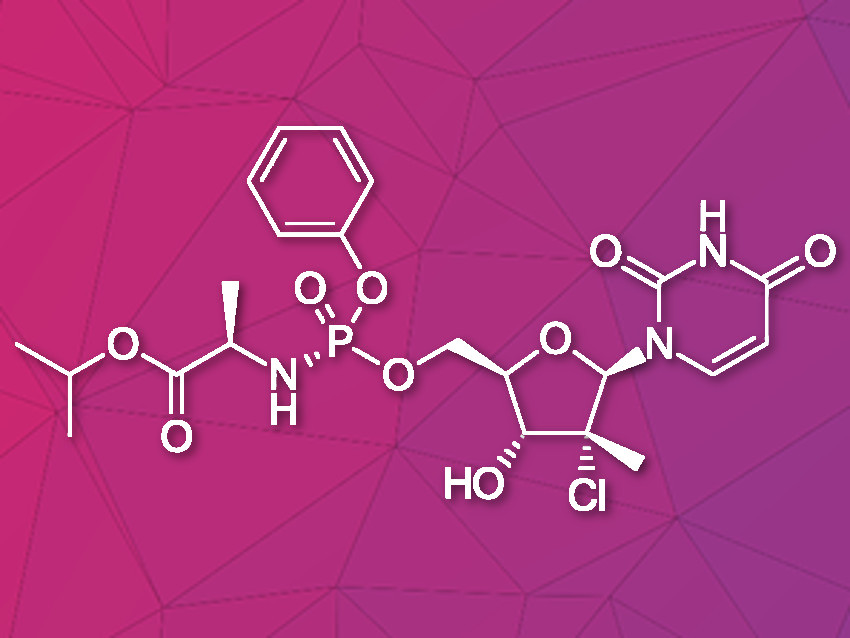
Uprifosbuvir is an antiviral agent developed for treatment of chronic hepatitis C infections. Its original synthesis route requires twelve steps with an overall yield of only 1 %. Such a difficult and time-consuming synthesis approach is acceptable for the early trial phase of a new drug, but impractical for broad application as hepatitis C treatment or for repurposing against novel viral diseases.
Artis Klapars, John Y. L. Chung, and colleagues, Merck & Co., Inc., Rahway, NJ, USA, and WuXi STA, Shanghai, China, have developed a synthesis route for uprifosbuvir requiring only five steps and starting from readily available uridine. Initially, uridine is selectively oxidized after OH-acylation with pivaloyl chloride in an acyl migration/oxidation process driven by complexation with the Lewis acid BF3*OEt2 in toluene. In the second step, methylation is achieved by MeMgBr/MgCl2 in a toluene/anisole mixture where a more reactive methyl-manganese species is formed in-situ from the Grignard reagent, providing high yield and a good diastereomeric ratio (dr). Subsequently, the tertiary chloride group is introduced. Due to the high functional-group density, a cyclodehydration step is required before chlorination to avoid side reactions. The chlorination is carried out using dichlorodimethylsilane with FeCl3*6H2O and tetramethyldisiloxane as additives which avoids the hazardous use of HCl gas under pressure required in the initial synthesis. In the final step, the regioselective phosphoramidation is achieved using a chlorophosphoramidate precursor and a dimeric chiral imidazole carbamate catalyst which led to a dr of 97:3 starting from a 1:1 diastereomeric mixture of the chlorophosphoramidate reagent.
Uprifosbuvir was synthesized with an overall yield of 50 %, a vast improvement compared to the 1 % of the original synthesis route. Additionally, the newly developed synthesis steps have the potential to provide easier access to other nucleoside-based antiviral agents.
- Efficient Synthesis of Antiviral Agent Uprifosbuvir Enabled by New Synthetic Methods,
Artis Klapars, John Chung, John Limanto, Ralph Calabria, Louis-Charles Campeau, Kevin Campos, Wenyong Chen, Stephen M Dalby, Tyler A Davis, Daniel DiRocco, Alan Hyde, Amude M Kassim, Mona Utne Larsen, Guiquan Liu, Peter Maligres, Aaron Moment, Feng Peng, Rebecca Ruck, Michael Shevlin, Bryon L Simmons, Zhiguo Jake Song, Lushi Tan, Timothy J Wright, Susan Zultanski,
Chemical Science 2021.
https://doi.org/10.1039/D1SC01978C