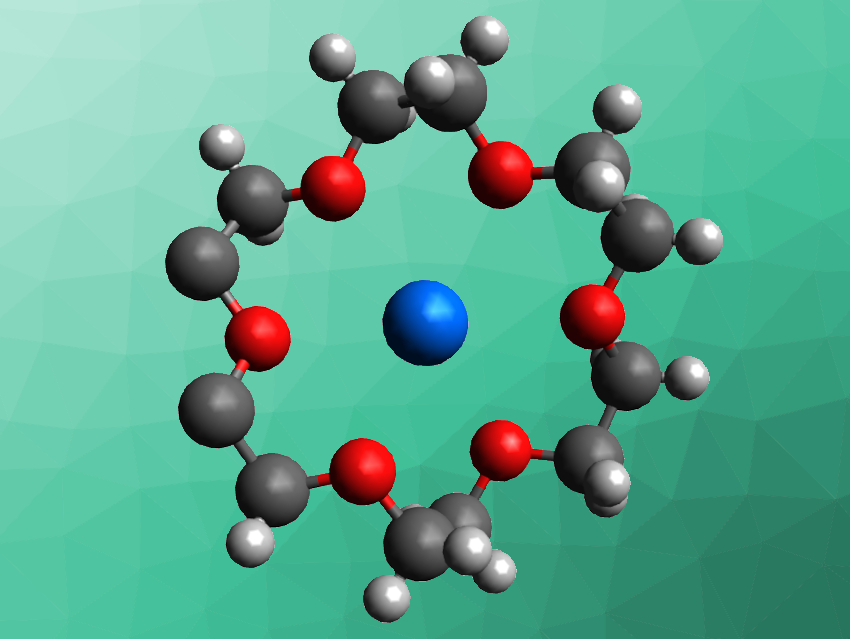
The coordination chemistry of actinides is important, e.g., in the processing of spent nuclear fuel, environmental cleanup applications, or nuclear medicine. Data on complexes of trivalent transuranic elements, however, is comparatively sparse. For exmaple, only a few trivalent transuranic complexes with macrocyclic ligands, such as crown ethers, are known.
Yaxing Wang, Soochow University, Suzhou, China, and colleagues have synthesized the first trivalent plutonium crown ether inclusion complex, [(H3O)(18-crown-6)][Pu(H2O)4(18-crown-6)](ClO4)4·2 H2O (partial structure pictured). The team first prepared a solution of PuVIO2(ClO4)2 and used H2O2 to reduce Pu(VI) to Pu(IV), followed by hydrobromic acid to reduce Pu(IV) to Pu(III). The resulting Pu(III) solution was combined with a solution of the crown ether 18-crown-6 in methanol and the solvent was evaporated to obtain the desired complex in the form of blue crystals.
The researchers used X-crystallography and solid-state UV–Vis–NIR spectroscopy to characterize the complex, as well as density functional theory (DFT) calculations to investigate its electronic properties. They found that the compound crystallizes in the orthorhombic space group Pccn. The plutonium atom is coordinated by ten oxygen atoms, six from the 18-crown-6 ligand and four from water molecules. The results of the calculations indicate that there is weak Pu–O dative bonding between the Pu(III) ion and the crown ether. According to the team, the work may help to improve the understanding of host–guest interactions between trivalent transuranic elements and macrocyclic ligands.
- Synthesis and Characterizations of a Plutonium(III) Crown Ether Inclusion Complex,
Kai Li, Shuxian Hu, Qing Zou, Yugang Zhang, Hailong Zhang, Yuan Zhao, Tong Zhou, Zhifang Chai, Yaxing Wang,
Inorg. Chem. 2021.
https://doi.org/10.1021/acs.inorgchem.1c00886