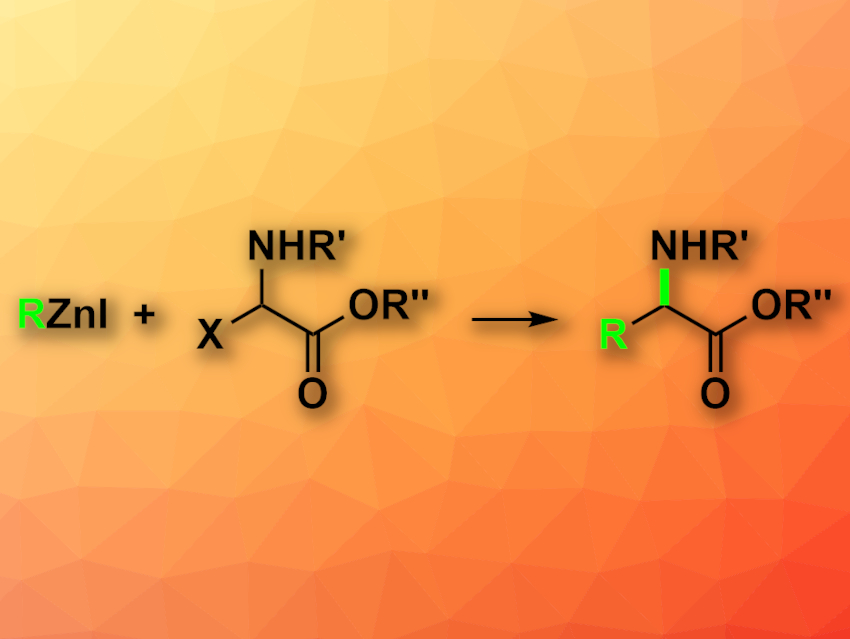
Unnatural α-amino acids, i.e., those not found in natural proteins, can be useful, e.g., in medicinal chemistry, biochemistry, or materials science. Thus, methods for the efficient enantioselective synthesis of these amino acids are useful.
Gregory C. Fu, California Institute of Technology (Caltech), Pasadena, USA; and colleagues have developed an enantioconvergent coupling of racemic alkyl electrophiles with alkylzinc reagents (pictured), promoted by earth-abundant nickel. The team used a variety of different organozinc reagents and racemic electrophiles such as carboxybenzyl (Cbz)-protected α-haloglycine esters as coupling partners, NiBr2·glyme (glyme = dimethoxyethane) together with a chiral ligand (2,6-bis((4S,5R)-4-isopropyl-5-phenyl-4,5-dihydrooxazol-2-yl)pyridine) as the catalyst, and tetrahydrofuran (THF) as the solvent. The reactions were performed at 0 °C.
The desired protected unnatural α-amino acids were obtained in good yields and enantioselectivities. The reaction is performed under mild conditions and tolerates air and moisture, as well as a wide range of functional groups. The researchers demonstrated the usefulness of the approach by synthesizing amino acids that serve as intermediates in the synthesis of known bioactive compounds, such as the antimalarial apicidin A.
- Asymmetric Synthesis of Protected Unnatural α-Amino Acids via Enantioconvergent Nickel-Catalyzed Cross-Coupling,
Ze-Peng Yang, Dylan J. Freas, Gregory C. Fu,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c03903