Phosphonate derivatives have many applications in chemistry as well as neighboring fields. For example, they are used as flame retardants, often display promising biological activity, serve as important synthetic intermediates, and have a wide range of uses in catalysis and materials science.
Alkenylphosphonates are phosphonate derivatives where the phosphorus atom is bound to an sp2-hybridized carbon atom of a C=C double bond. Such molecules are usually synthesized by Pd-catalyzed cross-coupling reactions. However, these reactions are limited to suitable cross-coupling substrates and the palladium catalysts are expensive. Enone-substituted alkenyl phosphonates are not accessible by this method and previously required complex multi-step syntheses.
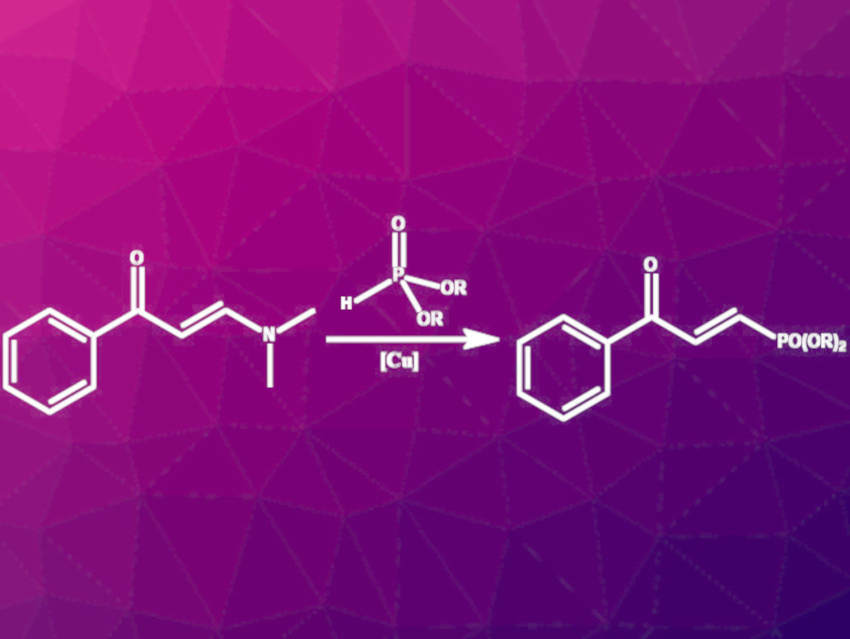
Yunyun Liu, Jie-Ping Wan, Jiangxi Normal University, Nanchang, China, and colleagues have developed a synthesis route for enone-substituted alkenylphosphonates via C–N-bond phosphonation of enaminones with dialkyl phosphites catalyzed by Cu(II). Commercially available and air-stable Cu(OTf)2 was used as the catalyst and p-toluenesulfonic acid (p-TSA) as an additive. The reaction (pictured) was performed in acetonitrile at 80 °C for 12 h under ambient air.
The reaction gave moderate to good yields for a wide range of enaminones with differently substituted phenyl groups. Furyl- and thiophenyl-derived enaminones were also successfully converted to the respective E-alkenyl phosphonates. Enaminones with N-heterocyclic or aliphatic residues did not react to the desired products. The reaction allows the introduction of diverse substituents at phosphorus. The products were successfully used in hydroamination, hydrothiolation, and sulfonyl hydrazone synthesis reactions, showing their potential as intermediates in the synthesis of more complex molecules.
- Copper-Catalyzed Enaminone C(sp2)–N Bond Phosphonation for Stereoselective Synthesis of Alkenylphosphonates,
Ting Liu, Li Wei, Baoli Zhao, Yunyun Liu, Jie-Ping Wan,
J. Org. Chem. 2021.
https://doi.org/10.1021/acs.joc.1c00862