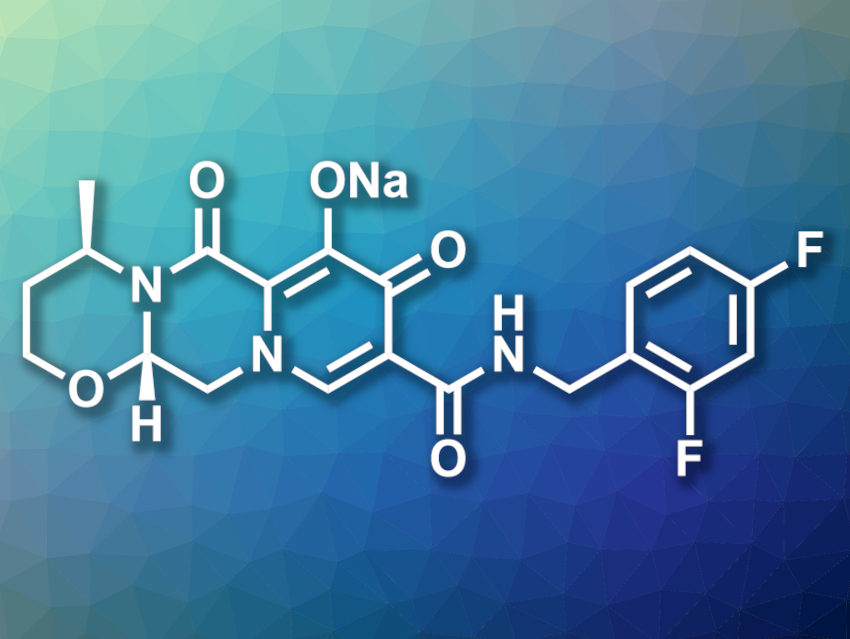
Dolutegravir is an antiretroviral drug used in the treatment of HIV/AIDS, usually in the form of its sodium salt (pictured). It acts as an integrase strand transfer inhibitor (INSTI), i.e., it interferes with the HIV integrase enzyme and prevents it from inserting viral DNA into the human genome. In existing syntheses of this drug, the pyridone ring is generally constructed first and then undergoes cyclization with (R)-3-amino-1-butanol to form additional rings.
Till Opatz, Johannes Gutenberg University, Mainz, Germany, and colleagues have developed an alternative, practical synthesis of dolutegravir sodium that starts from (R)-3-amino-1-butanol. They developed a four-step process that forms a bicyclic amine fragment from this starting compound via an N-acylation, an N-alkylation, and an intramolecular transacetalization. Then, the pyridone ring was formed using a 1,4-addition with an enol ether and a regioselective cyclization. Finally, the 2,4-difluorobenzylamine group was introduced to give dolutegravir, which was converted to the sodium salt.
The team obtained dolutegravir sodium in an isolated yield of up to 51 % over six linear steps. The synthesis was performed on a gram scale. According to the researchers, the developed synthetic route could be useful in industry. This might help to ensure the supply of this important antiretroviral drug.
- Six-Step Gram-Scale Synthesis of the Human Immunodeficiency Virus Integrase Inhibitor Dolutegravir Sodium,
Jule-Philipp Dietz, Tobias Lucas, Jonathan Groß, Sebastian Seitel, Jan Brauer, Dorota Ferenc, B. Frank Gupton, Till Opatz,
Org. Process Res. Dev. 2021.
https://doi.org/10.1021/acs.oprd.1c00139