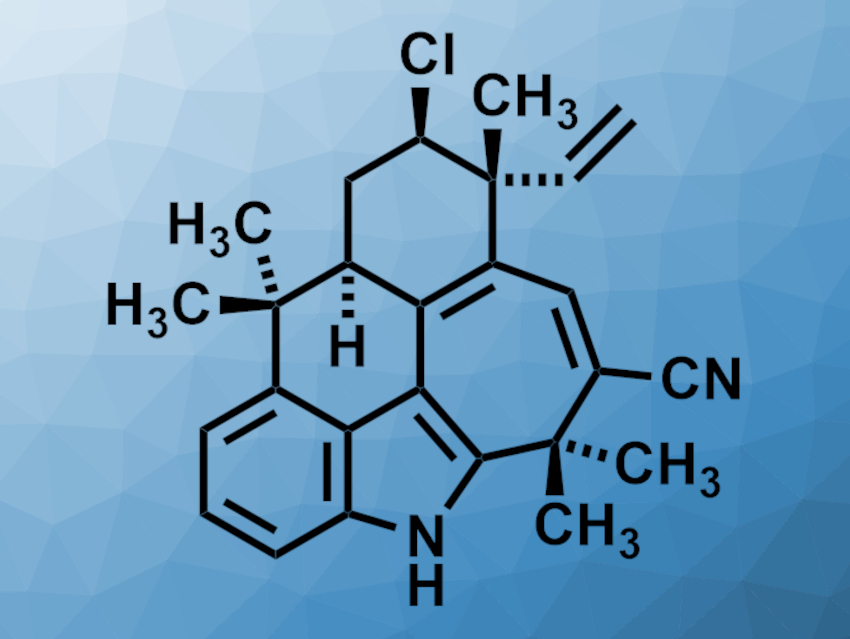
Ambiguines are indole alkaloid natural products first isolated from cyanobacteria. Some of these compounds have shown interesting bioactivities, e.g., antibacterial, antifungal, and cytotoxic properties. The complex structure of some ambiguines makes them challenging targets for total synthesis.
Lingbowei Hu and Viresh H. Rawal, University of Chicago, IL, USA, have performed a total synthesis of (+)-ambiguine G (pictured), the first chlorinated pentacyclic ambiguine to be synthesized in the laboratory. The team started from (S)-carvone oxide, which was converted to a chlorinated cyclohexanone in two steps. This compound was converted to an ethoxy diene and subjected to a cycloaddition with an indolic silyl ether to introduce the indole unit and form the seven-membered ring of the target compound. The resulting tetracyclic intermediate was converted to a pentacyclic product via an intramolecular Friedel–Crafts reaction. With the pentacyclic skeleton of (+)-ambiguine G fully formed, the team used a one-pot reduction–elimination–oxidation sequence and the introduction of a nitrile group by a palladium-catalyzed coupling reaction to obtain the target compound.
The researchers obtained (+)-ambiguine G as a single diastereomer in ten steps from (S)-carvone oxide using a convergent synthesis strategy. According to the team, the developed synthesis route could also provide access to other pentacyclic ambiguines and their analogues.
- Total Synthesis of the Chlorinated Pentacyclic Indole Alkaloid (+)-Ambiguine G,
Lingbowei Hu, Viresh H. Rawal,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c05762