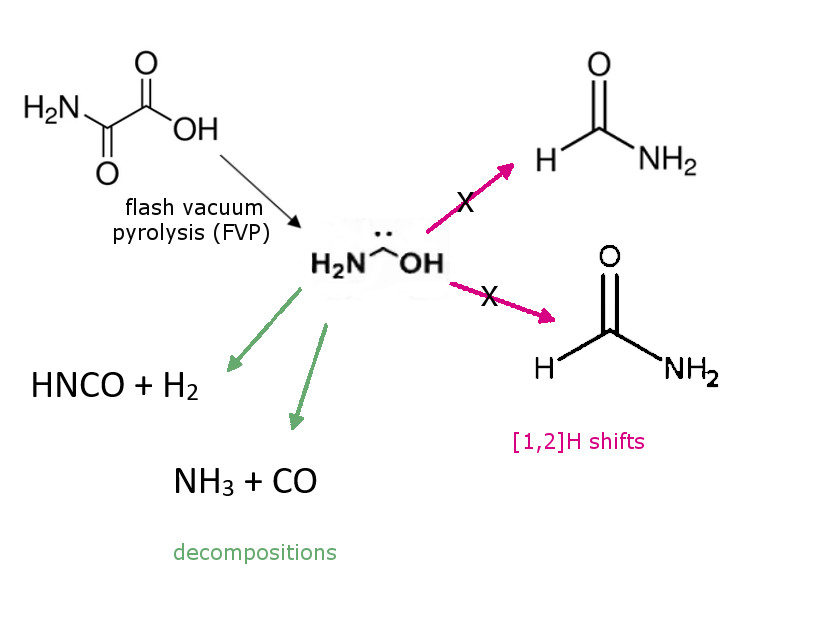
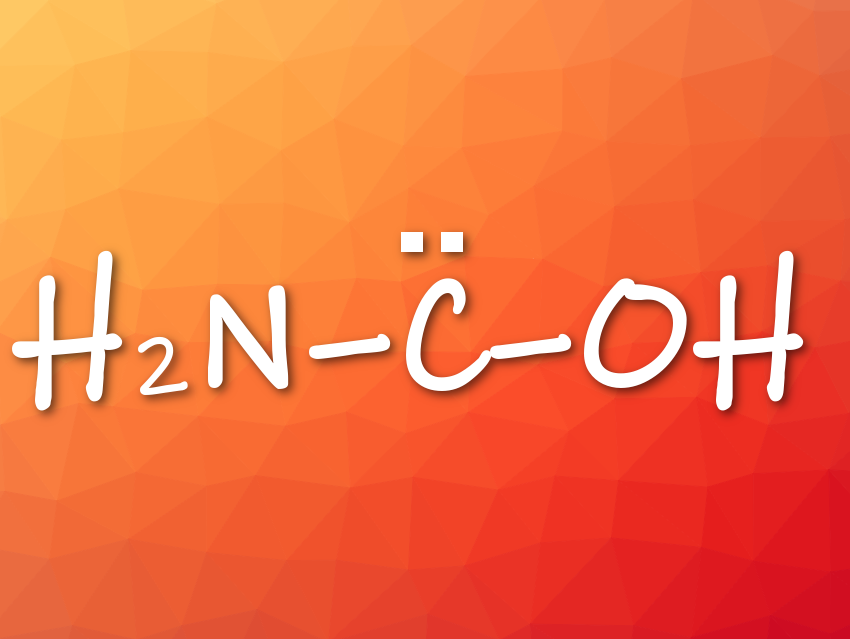Peter R. Schreiner and colleagues, Justus Liebig University, Gießen, Germany, have generated, isolated, and characterized the previously unknown aminohydroxymethylene (aminohydroxycarbene) in solid Ar via pyrolysis of oxalic acid monoamide. Aminohydroxymethylene is the simplest aminooxycarbene, an isomer of formamide and formimidic acid, and a prototypical electron donor-stabilized carbene. It is astrochemically relevant.
The researchers found that it is stable under cryogenic conditions and only decomposes to HNCO + H2 and NH3 + CO when the trans-conformer is irradiated at 254 nm. This result is in contrast to other hydroxycarbenes and aminomethylene, which undergo [1,2]H shifts to the corresponding carbonyls or imine. Due to very significant heteroatom stabilization, quantum mechanical tunneling (QMT) from the trans-conformer to formamide or formimidic acid does not take place on laboratory time-scales but may be relevant in astrochemical processes according to the researchers. The experimental data are well supported by the results of CCSD(T)/cc-pVTZ and B3LYP/6-311++G(3df,3pd) computations.
- Aminohydroxymethylene (H2N–C̈–OH), the Simplest Aminooxycarbene,
Bastian Bernhardt, Marcel Ruth, Hans Peter Reisenauer, Peter R. Schreiner,
J. Phys. Chem. A 2021.
https://doi.org/10.1021/acs.jpca.1c06151