Aryl halides are important intermediates in many chemical transformations. While bromides and iodides are often more reactive, chlorides are more stable and more easily accessible in large quantities.
Reactions of carbon-centered radicals generated by photoredox catalysis are an important method for the formation of new chemical bonds. So far, aryl chlorides have rarely been used in this kind of reaction due to the high C–Cl bonding energy, which makes activation by visible light very difficult. One approach to overcome this challenge is the use of a suitable organic photocatalyst in combination with a sacrificial reductant. These form a long-lived radical anion upon irradiation with visible light which is excited by a second photon to generate a more active reducing species. This process is referred to as consecutive photoinduced electron transfer (ConPET).
Rong Zhou, Taiyuan University of Technology, China, Jie Wu, National University of Singapore, and colleagues have found that 1,2,3,5-tetrakis(carbazol-9-yl)-4-6-dicyanobenzene (4CzIPN) can undergo ConPET, achieving reduction potentials sufficient for the activation of various aryl chlorides under visible light. This enables, e.g., borylation reactions of chloroarenes. The reactions were carried out in acetonitrile with 4-cyanopyridine as an additive, potassium phosphate as a base, and sodium oxalate as a sacrificial electron donor under blue LED light.
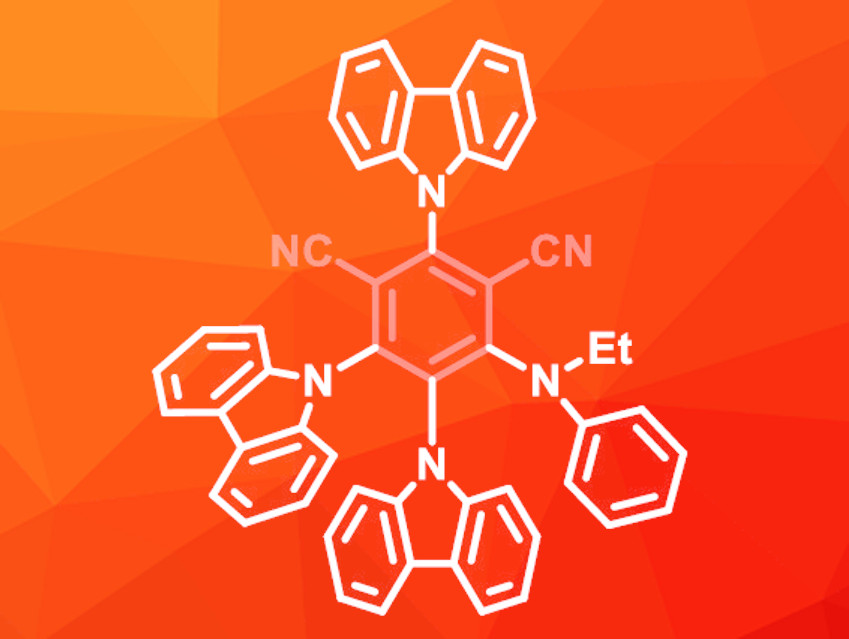
Due to the low stability of 4CzIPN during the reaction, the team synthesized new donor–acceptor dicyanobenzenes. In these molecules, one of the carbazole groups was replaced with an amine to improve the stability. The best results were achieved with an ethyl(phenyl)amino-substituted derivative (pictured), which gave the desired borylation product in an excellent yield of 95 %.
The reaction can be used with a wide range of activated and non-activated aryl chlorides and acetonitrile-soluble boron compounds. Phosphorylation of the carbon-centered radicals by phosphines or trimethylphosphite also proceeded smoothly, giving phosphonium salts and phosphonates in yields of 40–90 %. A phosphonium salt synthesis was also successfully carried out in a microtubing flow reactor on a gram scale.
- Unveiling Extreme Photoreduction Potentials of Donor-Acceptor Cyanoarenes to Access Aryl Radicals from Aryl Chlorides,
Jinhui Xu, Jilei Cao, Xiangyang Wu, Han Wang, Xiaona Yang, Xinxin Tang, Ren Wei Toh, Rong Zhou, Edwin K. L. Yeow, Jie Wu,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c05994