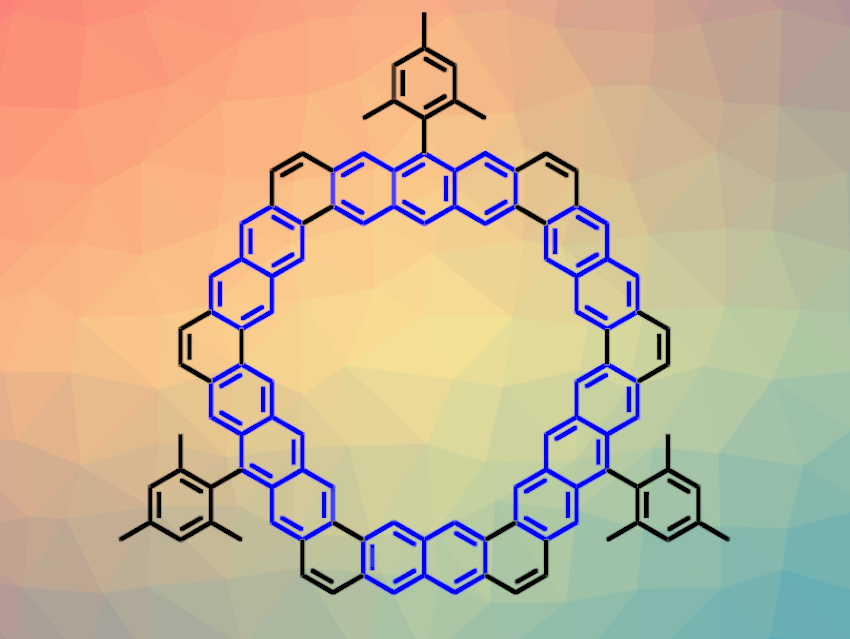
Kekulene is a cycloarene, i.e., a macrocycle composed of benzene units. Kekulene has a hexagonal structure with three benzene units at each edge. Extending these edges with further benzene rings would lead to [m,n]cycloarenes, with m and n equal to the numbers of benzene units on two adjacent edges. In this notation, kekulene derivatives are denoted as [3,3]cycloarenes.
Jishan Wu, National University of Singapore and Joint School of National University of Singapore and Tianjin University, Fuzhou, China, and colleagues have synthesized [m,n]cycloarene derivatives with extended edges, i.e., [3,4]-, [3,5]-, [4,4]-, and [4,5]cycloarenes (latter pictured above). The team first prepared suitable macrocyclic precursors functionalized with vinyl ethers using Suzuki coupling reactions. Then, they used a bismuth(III) triflate-catalyzed cyclization reaction to close the remaining six-membered rings and obtain the desired cycloarenes.
The products were characterized using X-ray crystallography and NMR spectroscopy, as well as density functional theory (DFT) calculations. The team found that the π-electrons in the compounds’ macrocyclic cores are mainly delocalized within individual benzene, naphthalene, or anthracene units at the edges (pictured in blue in the example above).
- Expanded Kekulenes,
Wei Fan, Yi Han, Xuhui Wang, Xudong Hou, Jishan Wu,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c06757