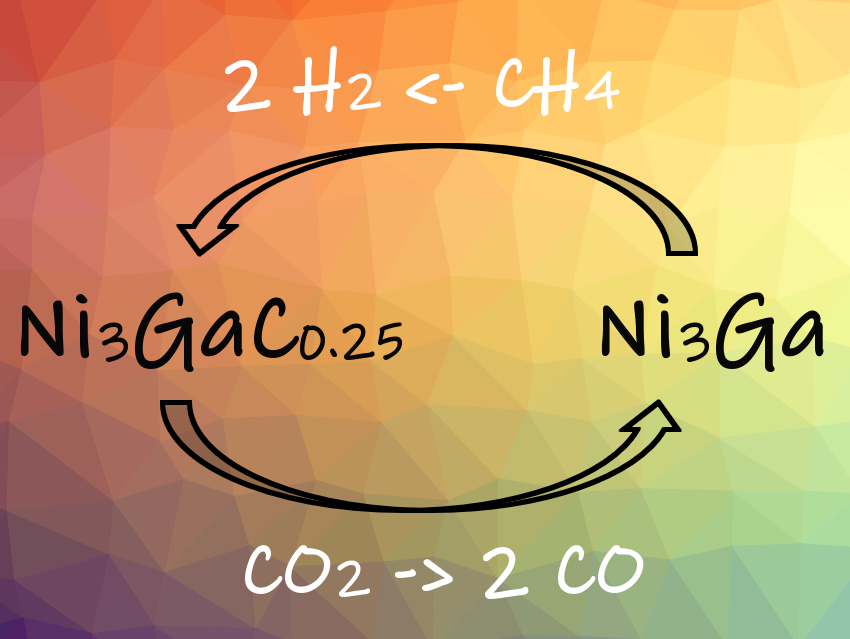Seok Ki Kim, Korea Research Institute of Chemical Technology (KRICT), Daejeon, Kwangjin An, Jae Sung Lee, and colleagues, Ulsan National Institute of Science and Technology (UNIST), Republic of Korea, have synthesized a NiMgGa layered double hydroxide (NMG-LDH) as a catalyst precursor for the dry reforming of methane.
The team dissolved nickel(II) nitrate hexahydrate [Ni(NO3)2-6H2O], magnesium(II) nitrate hexahydrate [Mg(NO3)2-6H2O], and gallium(III) nitrate hydrate [Ga(NO3)3-xH2O] in water. They added a solution containing sodium carbonate (Na2CO3) and sodium hydroxide (NaOH) in water. After stirring, the solution was sealed in an autoclave and kept at 120 °C for 24 hours. After cooling, a green precipitate was collected by filtration and dried in a vacuum oven. Samples of this NMG-LDH were reduced in 50 % H2/Ar at various temperatures. The obtained catalysts consisted of crystalline Ni3Ga nanoparticles (NPs) embedded in MgO nanosheets. Ni and Ga are uniformly distributed in the Ni3Ga NPs on the MgO support.
The researchers performed the dry reforming of methane in a fixed-bed quartz reactor. During the reaction, the reversible phase transition between Ni3Ga and Ni3GaCx activates both CO2 and CH4 (pictured). Interstitial carbon is an important reaction intermediate: Carbon atoms dissociated from CH4 enter the interstices of the Ni3Ga lattice during dry reforming of methane at 600 °C. By this, the Ni3Ga catalyst is converted into a Ni3GaC0.25 phase. This intermetallic carbide readily reacts with CO2 to form CO. This prevents the formation of coke on the catalyst surface and avoids deactivation of the catalyst by coking. The mechanism is unique in that the interstitial carbon becomes the reactive intermediate, whereas in most heterogeneous catalysts only surface intermediate species are involved.
The Ni3Ga/MgO catalyst shows ∼48 % CH4 and ∼52 % CO2 conversion and excellent stability against coking during dry reforming of methane. According to the researchers, their results provide valuable insights for preparing intermetallic catalysts with a high stability against coking.
- Layered Double Hydroxide-Derived Intermetallic Ni3GaC0.25 Catalysts for Dry Reforming of Methane,
Kwang Young Kim, Jin Ho Lee, Hojeong Lee, Woo Yeong Noh, Eun Hyup Kim, Eun Cheol Ra, Seok Ki Kim, Kwangjin An, Jae Sung Lee,
ACS Catal. 2021, 11, 11091–11102.
https://doi.org/10.1021/acscatal.1c02200