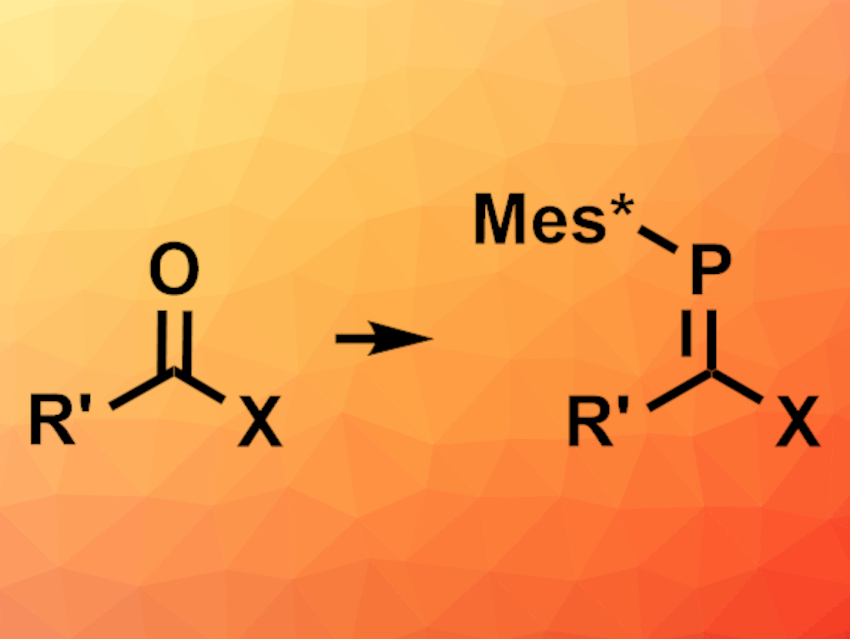
In the Wittig reaction, an aldehyde or ketone is converted to an alkene via a reaction with a phosphonium ylide. Phosphaalkenes (RR’C=PR”) are similar to alkenes. They are usually prepared via methods that require a preexisting P–C σ-bond. A reaction analogous to the Wittig reaction that forms the P=C bond and gives phosphaalkenes can be useful. “Phospha-Wittig” reagents for this type of reaction are available, but the reaction is less well-developed than the Wittig reaction, the reagents can be challenging to prepare, and the substrate scope can be limited.
Michael J. Cowley, University of Edinburgh, UK, and colleagues have developed a “phospha-bora-Wittig” reaction, in which the transient phosphaborene [Mes*P═BNR2] (Mes* = 2,4,6-tri-tert-butylphenyl; NR2 = 2,2,6,6-tetramethylpiperidine) reacts with carbonyl compounds to give phosphaalkenes of the type RR’C=PMes* (pictured). The phosphaborene is generated in situ from a dimeric diphosphadiboretane precursor, which was reacted with different aldehydes, ketones, esters, or amides in benzene at 80 °C to give the corresponding formal [2+2] cycloaddition product, a 1,2,3-phosphaboraoxetane. The addition of AlBr3 then led to the desired ring-opening, gave the corresponding phosphaalkenes, and released [R2NBO]n as a byproduct.
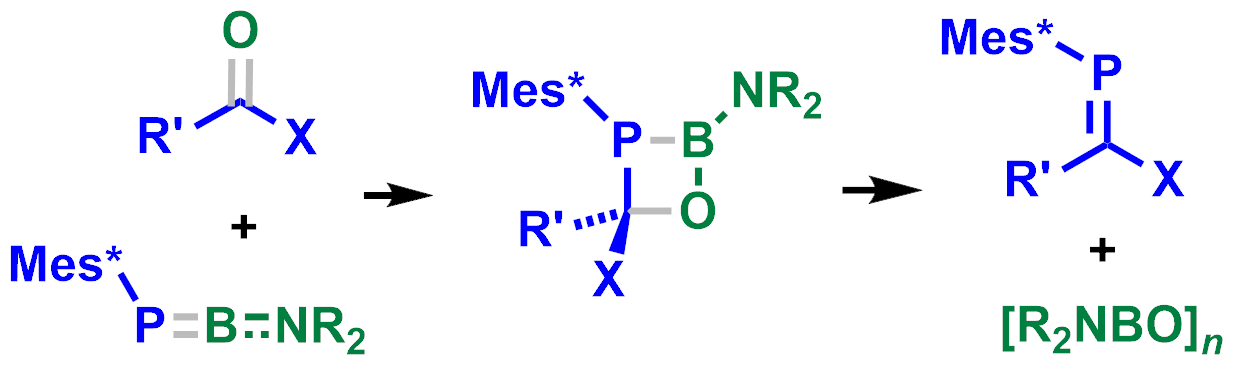
The developed reaction provided access to known and new phosphaalkenes and, thus, could extend the scope of “phospha-Wittig”-type reactions. According to the researchers, they are working on extending the scope to include reactants with other substituents at phosphorus.
- The Phospha-Bora-Wittig Reaction,
Andryj M. Borys, Ella F. Rice, Gary S. Nichol, Michael J. Cowley,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c06228