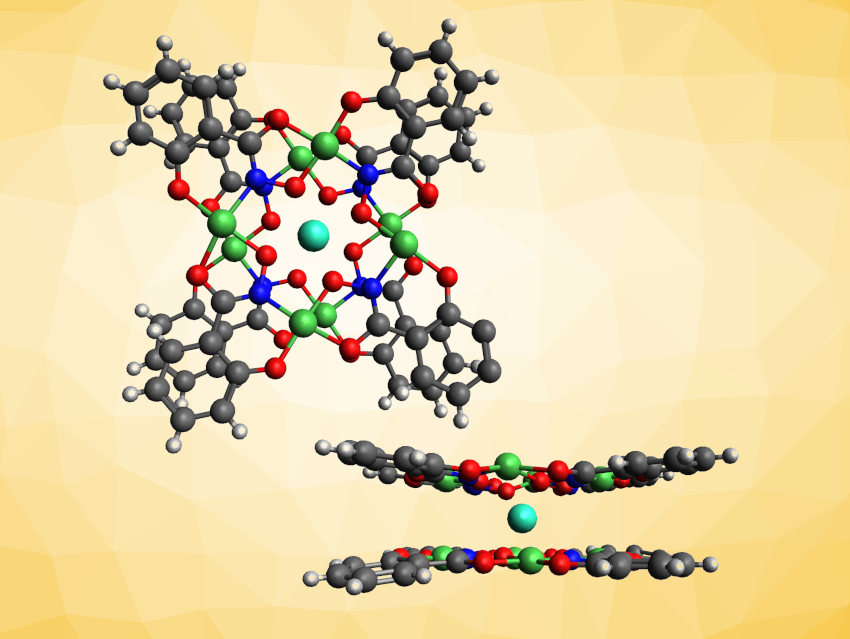
Single-molecule magnets (SMMs) could be useful, e.g., for high-density data storage. Lanthanide-based SMMs—in particular, double-decker complexes—have shown promise and can provide large energy barriers for the relaxation of magnetization. The symmetry of the coordination sphere around the lanthanide ion influences the properties of the resulting SMM. Metallacrowns (MCs) are macrocyclic ligands that could be useful to design a suitable coordination environment. They are structurally similar to crown ethers but contain metal atoms in their ring.
Eva Rentschler, University of Mainz, Germany, and colleagues have synthesized a new lanthanide double-decker complex with nickel metallacrowns (MCs) as coordinating ligands (top view and side view pictured). The team prepared the complex of the type TbIII[12-MCNiIIN(shi)-4]2 (shiH3 = salicylhydroxamic acid) using a reaction of terbium(III) pivalate with salicylhydroxamic acid, nickel(II) pivalate, and piperidine in acetone. The desired sandwich complex was obtained in a yield of ca. 25 % as a piperidine adduct. It was characterized using elemental analysis, single-crystal X-ray diffraction, and magnetic measurements.
The team found that in the metallacrowns, the Ni(II) ions are bridged by shi3– ligands, forming rings with four –Ni–N–O− units each. The central Tb(III) ions are coordinated to the oxygen atoms of two metallacrown ligands, forming a double-decker-type complex with a nearly ideal square-antiprismatic coordination sphere for the Tb(III) center. The complex shows an energy barrier of 346 K under zero field and up to 585 K under an applied magnetic field (3200 Oe). According to the researchers, this energy barrier is the highest reported so far for a metallacrown with D4d symmetry.
- 3d/4f Sandwich Complex Based on Metallacrowns,
Andreas Rauguth, Alexander Kredel, Luca M. Carrella, Eva Rentschler,
Inorg. Chem. 2021.
https://doi.org/10.1021/acs.inorgchem.1c01356