Cyano- and isocyanosilylenes (e.g., Si(CN)H and Si(NC)H) are highly reactive species found, for example, in interstellar chemistry. They are challenging to study due to their instability. Only some of their feasible isomers have been found, e.g., in argon matrices. Isomers of the type Si=C=N–H have remained elusive so far. Bulky N-heterocyclic carbenes (NHCs) have previously been used to stabilize other reactive, low-coordinate silicon compounds, and thus, allow their detailed investigation.
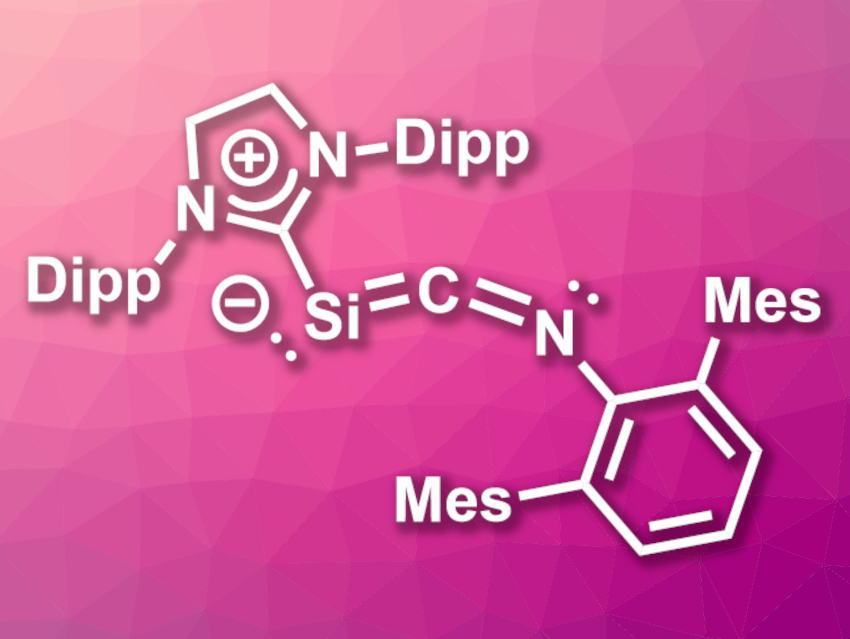
Ujjal Das, Alexander C. Filippou, University of Bonn, Germany, and colleagues have synthesized the first two-coordinate Si(0)-isocyanide compound, (SIDipp)Si=C=N–ArMes (pictured, SIDipp = C[N(Dipp)CH2]2, Dipp = 2,6-diisopropylphenyl, ArMes = 2,6-dimesitylphenyl), which is stabilized by an NHC. The team prepared this compound from the isocyanide-stabilized silyliumylidene salt [(SIDipp)SiBr(CNArMes)][B(ArF)4] (ArF = B(C6H3-3,5-(CF3)2)4), which was reduced using KC8. The silyliumylidene salt was synthesized from (SIDipp)SiBr2 via a bromide abstraction using Na[B(ArF)4] and CNArMes.
The product was fully characterized and the team found that it contains a silacumulene-type, slightly bent Si=C=N core, and has a bond angle at the dicoordinate silicon of ca. 100°. It is axially chiral. Reactivity studies showed that (SIDipp)Si=C=N–ArMes can be used to transfer an Si0(NHC) unit, e.g., to the germanium compound Ge(ArMes)Cl, which results in an NHC-stabilized germasilyne of the type (NHC)–Si(Cl)=Ge–ArMes. This synthetic potential could be useful in inorganic chemistry.
- (NHC)Si═C═N–R: A Two-Coordinated Si0-Isocyanide Compound as Si(NHC) Transfer Reagent,
Surendar Karwasara, Leonard R. Maurer, Benjamin Peerless, Gregor Schnakenburg, Ujjal Das, Alexander C. Filippou,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c06628