Hafnium is the heaviest stable group 4 transition metal. Complexes of the lighter group 4 elements titanium and zirconium have applications, e.g., in catalysis. The use of hafnium compounds is much less common even though hafnium compounds can be useful for bond-activation reactions. Titanium and zirconium are well known for the formation of titanocene and zirconocene complexes, but only relatively few corresponding hafnocene complexes have been described so far.
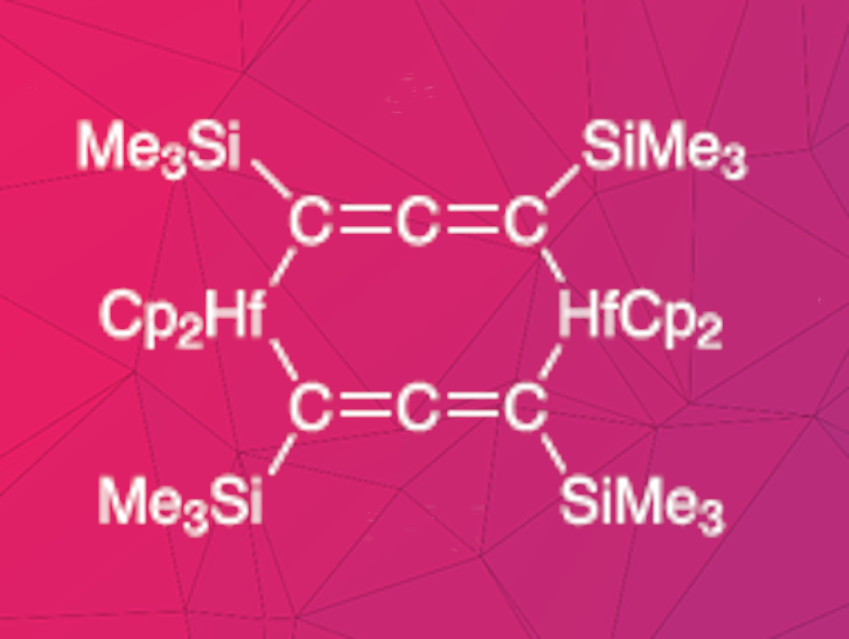
Fabian Reiß, Torsten Beweries, Leibniz Institute for Catalysis, Rostock, Germany, and colleagues have synthesized two dinuclear, allenediide-bridged hafnocene(IV) complexes. Cp2HfCl2 and the dilithioallene precursor Li2(Me3SiC3SiMe3) were reacted in toluene at –78 °C under a nitrogen atmosphere. The products were characterized using NMR spectroscopy, Fourier-transform infrared spectroscopy (FT-IR), and X-ray diffraction. Depending on the stoichiometry, two different complexes were formed. Two equivalents of the hafnium compound and one equivalent of the allenediide precursor gave a complex containing one bridging allenediide group and two chloride ligands. Equimolar amounts of both precursors led to a product with two bridging allenediide ligands (pictured).
The properties of the two complexes differ considerably. The mono-allenediide complex is air- and moisture-sensitive and was isolated in a yield of 21 %. The complex with two alllenediide bridges was isolated in a yield of 90 %. It is stable in air and various solvents, including water. The researchers attribute this high stability to the shielding effects of the cyclopentadienyl and trimethylsilyl units surrounding the metal center.
- Synthesis and Characterization of Dinuclear Allenediide Bridged Hafnocene(IV) Complexes,
Kevin Lindenau, Edgar Zander, Claas Schünemann, Anke Spannenberg, Maxim V. Andreev, Vladimir V. Burlakov, Fabian Reiß, Torsten Beweries,
Organometallics 2021.
https://doi.org/10.1021/acs.organomet.1c00385