Lithium is widely used in modern battery technologies. However, natural resources of lithium are limited, and its extraction can be expensive and time-consuming. Improved methods for the extraction of lithium from brines, which contain a large part of the world’s lithium resources, would be useful.
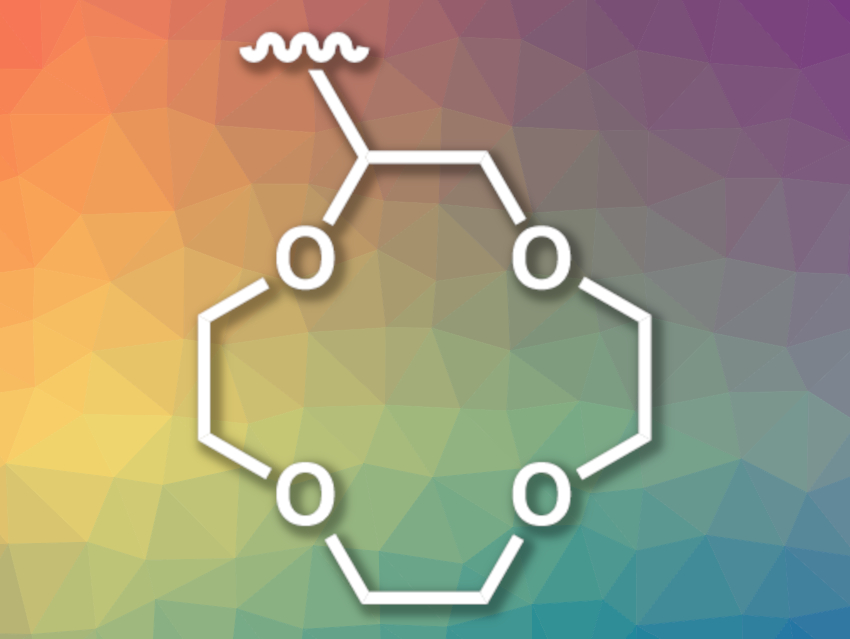
Polymer membranes could be used to separate lithium from other components of such brines, but achieving the required cation selectivity for Li+ over other ions such as Na+, K+, Ca2+, and Mg2+ is challenging—in particular, for other monovalent ions. The incorporation of ligands that bind these ions, e.g., crown ethers such as 12-crown-4 (pictured), could help to solve this problem.
Venkat Ganesan, Benny D. Freeman, The University of Texas at Austin, USA, Mahdi M. Abu-Omar, Christopher M. Bates, University of California, Santa Barbara, USA, and colleagues have developed polynorbornene networks that are functionalized with 12-crown-4 ether units to increase the transport selectivity of LiCl over NaCl. The team used three types of norbornene-based monomers to prepare the desired polymer: one that is functionalized with 12-crown-4 to provide ion selectivity, one with poly(ethylene oxide) to control the hydrophilicity, and thus, the water uptake, and one with a second norbornene-based group that acts as a crosslinker. The polymer membranes were then formed using a ring-opening metathesis polymerization (ROMP) in a solvent-casting approach.
The team found that, with an optimized water content in the membrane, the material provides a reverse permeability selectivity of about 2.3 for LiCl over NaCl. They attribute the effect to the higher affinity of the crown ether groups for Na+ under these conditions, i.e., 12-crown-4 binds Na+ ions more strongly than Li+, which reduces Na+ mobility. The work, thus, provides insights that could be useful for the design of membranes with ion-specific selectivity based on host–guest interactions.
- Engineering Li/Na selectivity in 12-Crown-4–functionalized polymer membranes,
Samuel J. Warnock, Rahul Sujanani, Everett S. Zofchak, Shou Zhao, Theodore J. Dilenschneider, Kalin G. Hanson, Sanjoy Mukherjee, Venkat Ganesan, Benny D. Freeman, Mahdi M. Abu-Omar, Christopher M. Bates,
Proc. Natl. Acad. Sci. USA 2021, 118, e2022197118.
https://doi.org/10.1073/pnas.2022197118