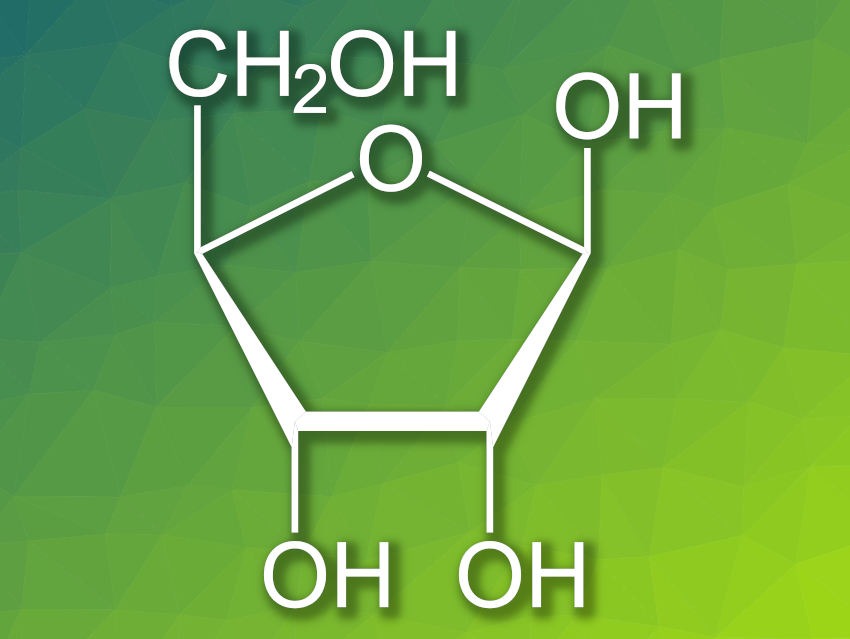
Ribose (pictured) is the sugar in ribonucleotides, which make up RNA. One hypothesis for the origin of life on Earth is that life began with self-replicating RNA molecules. However, ribose is just one of many similar sugars. How ribose was naturally selected as the only sugar in RNA is an open question. There are several theories of how ribose could have been chemically favored over other sugars—e.g., by stabilization as a boron complex. However, these theories make assumptions that might not necessarily have been true under prebiotic conditions.
Ze-Run Zhao and Xiao Wang, Nanjing University, China, have proposed a solution to this “ribose mystery” that circumvents the limitations of existing hypotheses. Instead of focusing on sugar synthesis, they propose ribose was selected via a separation process—similar to modern chromatography techniques. They separated a mixture of monosaccharides using ion- or ligand-exchange chromatography under different conditions and found that ribose was always the most strongly retained sugar among all pentoses.
The researchers propose that metal-doped clay minerals could have acted as a natural “stationary phase” and separated sugar mixtures formed on prebiotic Earth. They evaluated the retention behaviors of a mixture of monosaccharides on such minerals and found that ribose was the major sugar adsorbed. The retained ribose is stabilized, but still reactive enough for the subsequent synthesis of RNA. According to the researchers, the work could provide a plausible explanation for the natural selection of ribose.
- A plausible prebiotic selection of ribose for RNA – formation, dynamic isolation, and nucleotide synthesis based on metal-doped-clays,
Ze-Run Zhao, Xiao Wang,
Chem 2021.
https://doi.org/10.1016/j.chempr.2021.09.002