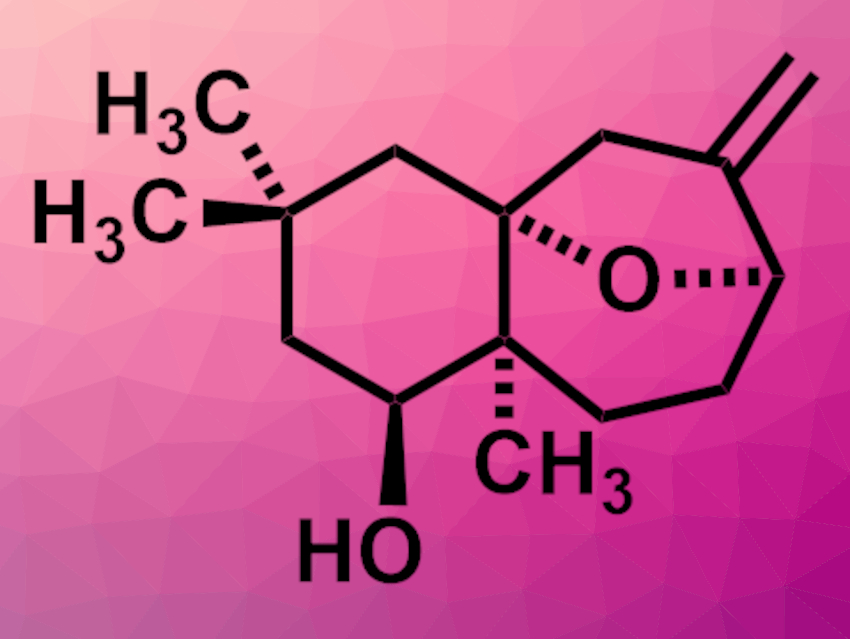
Toxicodendron vernicifluum, or the lacquer tree, is cultivated for its sap, which is used as a lacquer. The plant is also used in traditional herbal medicine. The sesquiterpenoids toxicodenane A (pictured), B, C, and E have been isolated from the plant. Toxicodenane A has an oxa-bridged tricyclic structure. Its relative stereochemical configuration has been determined, but its absolute configuration had not been assigned so far.
Fu-She Han, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, and University of Science and Technology of China, Hefei, and colleagues have performed the first enantioselective total synthesis of (+)-toxicodenane A and assigned its absolute configuration. The team started from a commercially available 1,3-cyclohexanedione, which was methylated and alkylated. An enantioselective reduction was then used to obtain a chiral 3-hydroxy ketone, followed by a Grignard reaction with prenylmagnesium bromide. The resulting intermediate was subjected to a transacetalation and Prins cyclization sequence to close the oxa-bridged ring system of toxicodenane. An ozonolysis, a Wittig reaction, and deprotection steps gave the desired (+)-toxicodenane A.
The team confirmed the structure of the product using NMR spectroscopy and high-resolution mass spectrometry (HRMS). The absolute configuration was assigned via a single-crystal X-ray analysis of the corresponding p-bromobenzoic ester. The synthesis has a longest linear sequence of nine steps and could allow the preparation of sufficient quantities of (+)-toxicodenane A for studies of its biological activities.
- Enantioselective Total Synthesis and Absolute Configuration Assignment of (+)-Toxicodenane A,
Xu-Long Qin, Guo-Jie Wu, Fu-She Han,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c03293