Oxidative dehydrogenative coupling reactions are atom-economical transformations in organic synthesis. However, they often require stoichiometric amounts of oxidants and/or harsh conditions. Autoxidative dehydrogenative coupling reactions using oxygen as the sole oxidant could be a more environmentally friendly alternative.
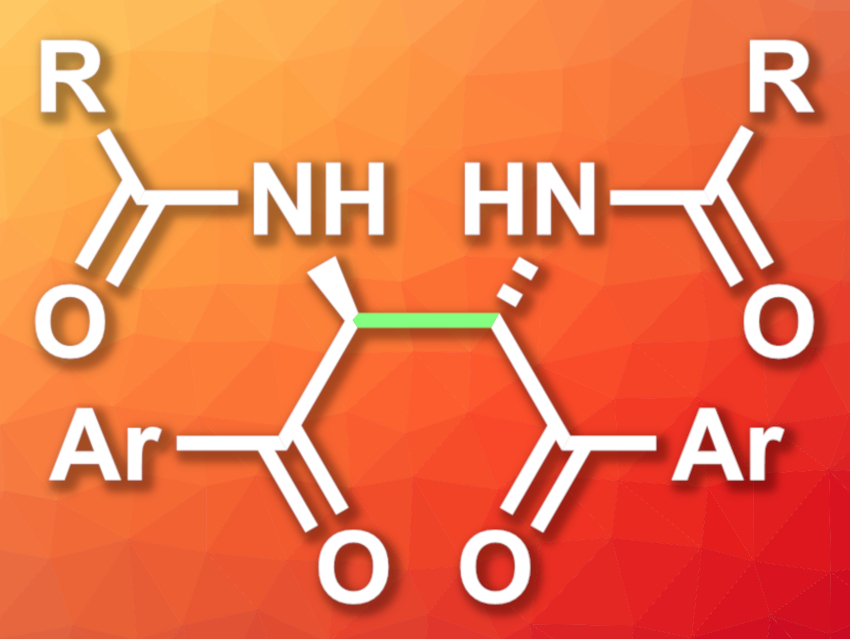
Zhihong Jiang, Macau University of Science and Technology, China, and colleagues have developed a method for the diastereoselective synthesis of 2,3-diamide-1,4-diones (general structure pictured) via an autoxidative dehydrogenative homocoupling of N-acyl-2-aminoacetophenones. The reaction is mediated by t-BuOK and was performed in dimethyl sulfoxide (DMSO) at room temperature under air.
The desired coupling products were obtained in generally moderate to good yields and good diastereoselectivities. The products are useful building blocks for further transformations. The team proposes a reaction mechanism that involves an SN2 pathway. First, a hydroperoxide is formed from the substrate and oxygen. Then, the NH group is deprotonated by t-BuOK to give a potassium imidate intermediate. A second equivalent of the substrate reacts with t-BuOK to form a nucleophile, which attacks the first intermediate and releases a hydroperoxide anion. Protonation then gives the desired product.
- Potassium-Base-Mediated Autoxidative Diastereoselective Homocoupling of N-Acyl-2-aminoacetophenones,
Yingwei Wang, Mingrong Yang, Chichou Lao, Zhihong Jiang,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00618