Synthesizing compounds with Al–Al bonds can be challenging due to their instability. Dianionic dialanes of the type [R3Al–AlR3]2– are particularly hard to access due to the electropositive nature of Al and strong electrostatic repulsion. Only a few examples exist, and none have a structure based on small rings.
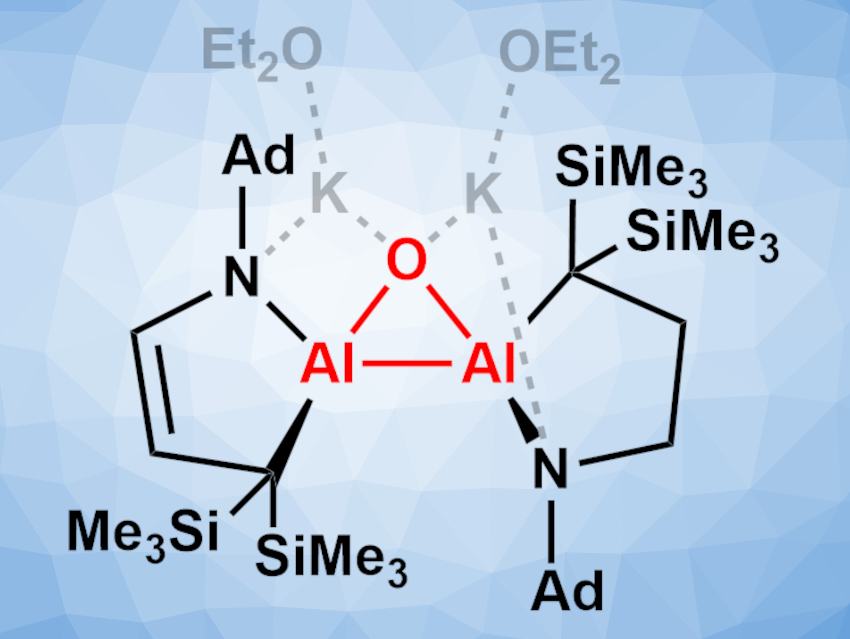
Kota Koshino and Rei Kinjo, Nanyang Technological University, Singapore, have synthesized a dianionic dialane with an Al2O three-membered ring—an aluminium analog of an epoxide (pictured, Ad = 1-adamantyl). The team prepared the compound by reducing the corresponding dialane with potassium graphite (KC8) in diethyl ether, followed by treatment with triethylphosphine oxide. The product was obtained as a yellow solid in a yield of 54 %.
The synthesized compound was characterized using X-ray crystallography. It features a three-membered Al2O ring and two five-membered rings and interacts with the counterions via the O and N atoms. The highly strained Al–Al bond is shorter than in other known dianionic dialanes. The product is sensitive toward moisture and oxygen and readily reacts with small molecules such as tert-butyl isocyanide, ethylene, or benzophenone to give Al-containing heterocycles.
- A Highly Strained Al–Al σ-Bond in Dianionic Aluminum Analog of Oxirane for Molecule Activation,
Kota Koshino, Rei Kinjo,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c07389