Aryl and heteroaryl methyl ethers are often found, e.g., in natural products or pharmaceutically active molecules. Frequently, they are synthesized from phenols or their equivalents. However, this can require harsh conditions or toxic reagents. While palladium-catalyzed C–O bond cross-couplings can be performed to react aryl halides with alcohols or alkoxides, this method is not ideal for the introduction of methoxy groups and the catalysts are expensive.
Chen Li, Wuyi University, Jiangmen, China, Dong-Hui Wang, Shanghai Institute of Organic Chemistry and Nanjing University of Chinese Medicine, both China, and colleagues have developed a copper-catalyzed cross-coupling reaction between aryl bromides and 9-methoxy-9-borabicyclo[3.3.1]nonane (9-BBN-OMe) to obtain aryl methyl ethers (pictured) under mild conditions. The team used CuBr as a catalyst, N,N‘-bis(4-hydroxyl-2,6-dimethylphenyl)oxalamide (BHMPO) as a ligand, Cs2CO3 as a base, and N-methyl-2-pyrrolidone (NMP) as the solvent. The reactions were performed at 80 °C.
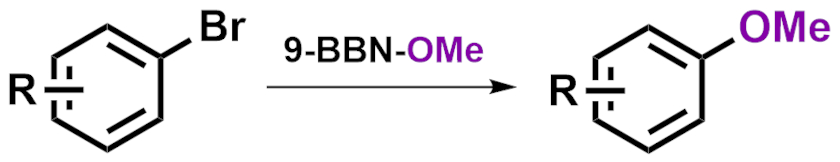
Under these conditions, the team converted a large variety of aryl bromides and heteroaryl bromides to the desired methoxy ethers in moderate to good yields. The reaction tolerates a wide range of functional groups.
- Copper-Catalyzed Methoxylation of Aryl Bromides with 9-BBN−OMe,
Jing-Ru Wang, Zhi-Qiang Song, Chen Li, Dong-Hui Wang,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c03172