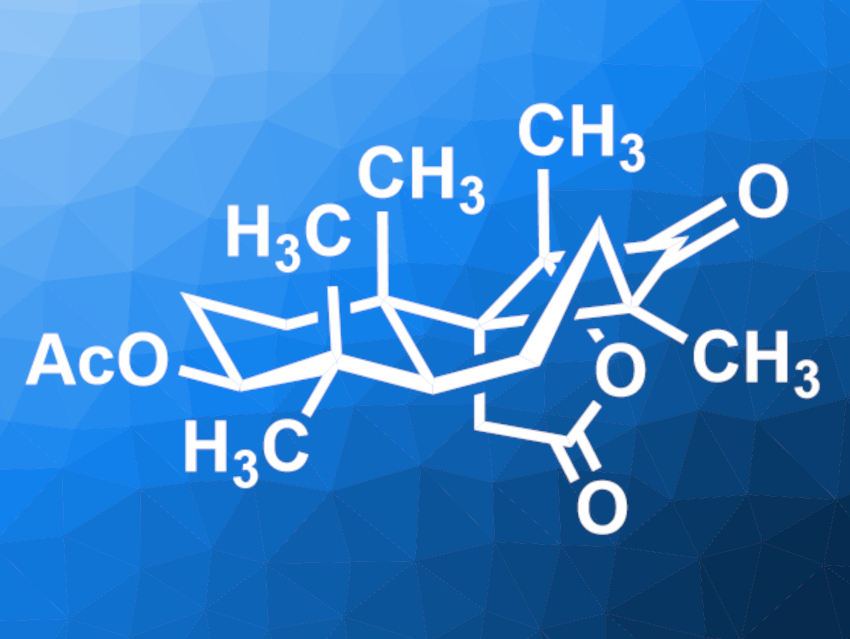
Cyclobutastellettolide B (pictured) was originally isolated from a Stelletta sea sponge. It is a potential lead compound for drug development. The compound has an unusual 6/6/4-fused tricyclic core with multiple quaternary stereocenters, which makes it a challenging target for total synthesis.
Jun Huang, Peking University Shenzhen Graduate School, Shenzhen, China, Zhen Yang, Peking University Shenzhen Graduate School, Shenzhen Bay Laboratory, and Peking University, Beijing, all China, and colleagues have performed a concise enantioselective total synthesis of (+)-cyclobutastellettolide B. The team started from commercially available geranyl acetate, which was converted to an epoxide intermediate in five steps. This intermediate was subjected to a polycyclization and then hydroxymethylated. A Johnson–Claisen rearrangement then gave the 6/6 ring system with a terminal alkene. This alkene group was converted to an aldehyde. A Wittig reaction and another oxidation step were used to transform this group to an α-diketone.
In a key step, the diketone was used in a Norrish–Yang photocyclization to form the four-membered ring of cyclobutastellettolide B. Finally, an intramolecular lactonization gave the desired product. Overall, the team obtained (+)-cyclobutastellettolide B in 13 steps from the epoxide in a total yield of 31.5 %.
- Total Synthesis of (+)-Cyclobutastellettolide B,
Zhongchao Zhang, Sijia Chen, Fu Tang, Kai Guo, Xin-Ting Liang, Jun Huang, Zhen Yang,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c08880