Perovskite or double perovskite structures are adopted by a variety of compounds. They can have a range of applications, e.g., in optoelectronics. The stability and band gap of the materials need to be optimized for the specific type of application. Oxide perovskites, for example, can show higher stabilities than halide perovskite compounds.
Optimal band gaps for, e.g., solar cell applications are often in the near-IR range. For achieving these bands gaps, the inclusion of heavier elements can be useful. For example, the band gap of the oxide double perovskite Ba2AgIO6 could be optimized by replacing Ag with the heavier Au. However, there had been no gold oxide perovskites of this type reported so far.
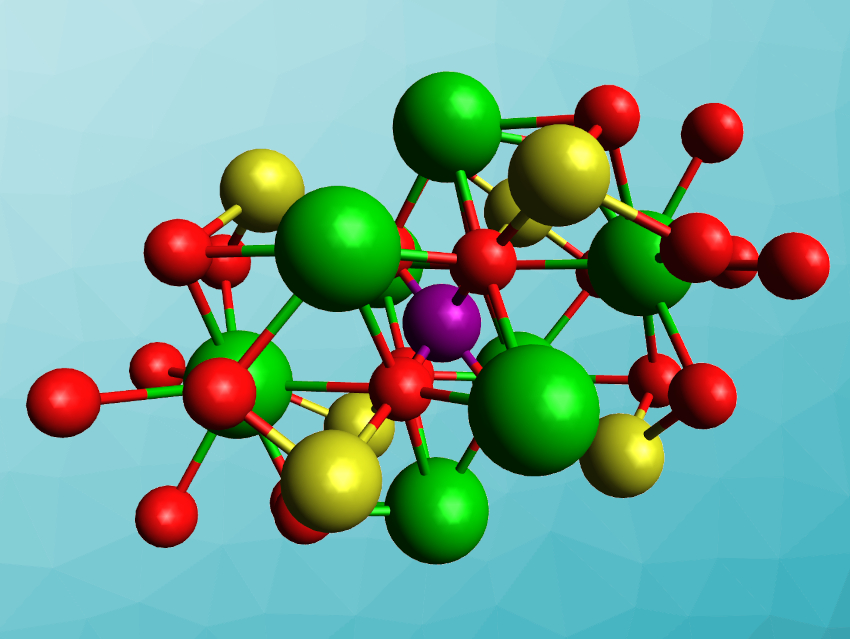
Tyrel M. McQueen, Johns Hopkins University, Baltimore, MD, USA, and colleagues have synthesized the first gold-containing double perovskite, Ba2AuIO6 (pictured) and compared it with the “lighter” analogues Ba2AgIO6 and Ba2NaIO6. The team used hydrothermal syntheses to prepare Ba2AgIO6 and Ba2AuIO6 from either Au2O3 or Ag2O, Ba(OH)2·8H2O, and H5IO6. Ba2NaIO6 was synthesized from NaIO4 and Ba(OH)2·8H2O via a solid-state reaction.
The products were characterized using X-ray diffraction, confirming the double-perovskite structure. Ba2NaIO6 has a cubic perovskite structure, while Ba2AuIO6 and Ba2AgIO6 show a monoclinic distortion. The band gaps of Ba2AuIO6 and Ba2AgIO6 are ca. 1.3 eV and ca. 2.1 eV, respectively. These values are promising for solar and other optoelectronic applications, but the stability of the compounds still needs improvement.
- A Gold(I) Oxide Double Perovskite: Ba2AuIO6,
Elizabeth A. Pogue, Jack Bond, Cassandra Imperato, John B. S. Abraham, Natalia Drichko, Tyrel M. McQueen,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c08241