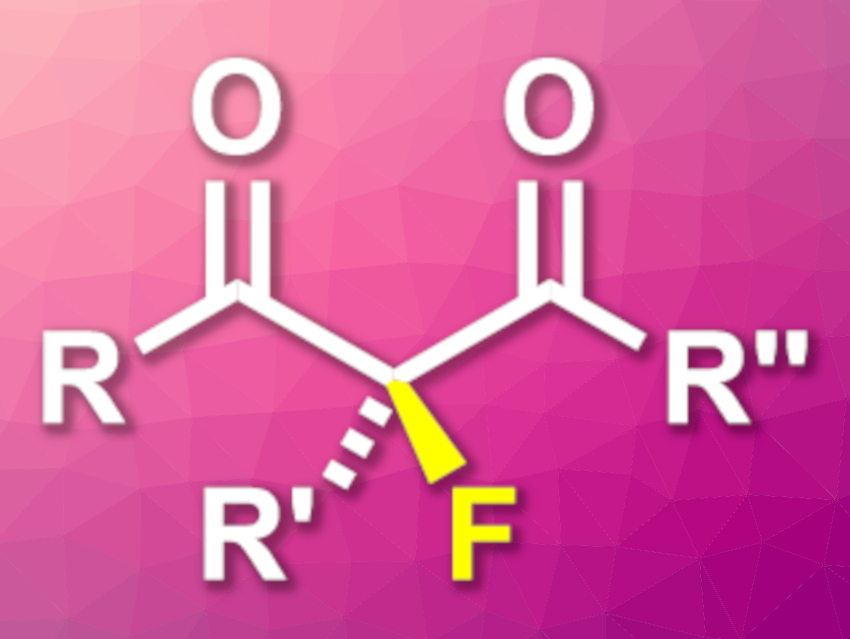
β-Dicarbonyl compounds are useful intermediates in organic synthesis. α-Substituted β-dicarbonyls, for example, can be used as substrates in asymmetric transformations. Fluorinated derivatives, in particular, can be useful in pharmaceutical chemistry due to the often positive effects of fluorination on biological activities. There are some examples of asymmetric fluorinations of α-substituted β-dicarbonyl compounds, e.g., β-ketoesters, but β-diketones can be challenging substrates for enantioselective transformations.
Satoru Arimitsu, University of the Ryukyus, Nishihara, Japan, and colleagues have developed a method for the enantioselective fluorination of α-substituted β-diketones (product pictured), using β,β-diaryl serines as an organocatalyst. The team used Selectfluor (1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) as a fluorinating reagent, a β,β-diaryl serine with bulky aryl substituents (i.e., 3,5,-(t-Bu)2-4-MeO-C6H2) as the catalyst, and tetrahydrofuran (THF) as the solvent. The reactions were performed at 40 °C.
Using this approach, the researchers converted a range of α-substituted β-diketones to the desired fluorinated products with high to excellent yields and enantioselectivities. The products can be further transformed, e.g., to fluorinated diols or aldols.
- Enantioselective Fluorination of α-Substituted β-Diketones Using β,β-Diaryl Serines,
Samira Poorsadeghi, Katsuki Endo, Satoru Arimitsu,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c04104