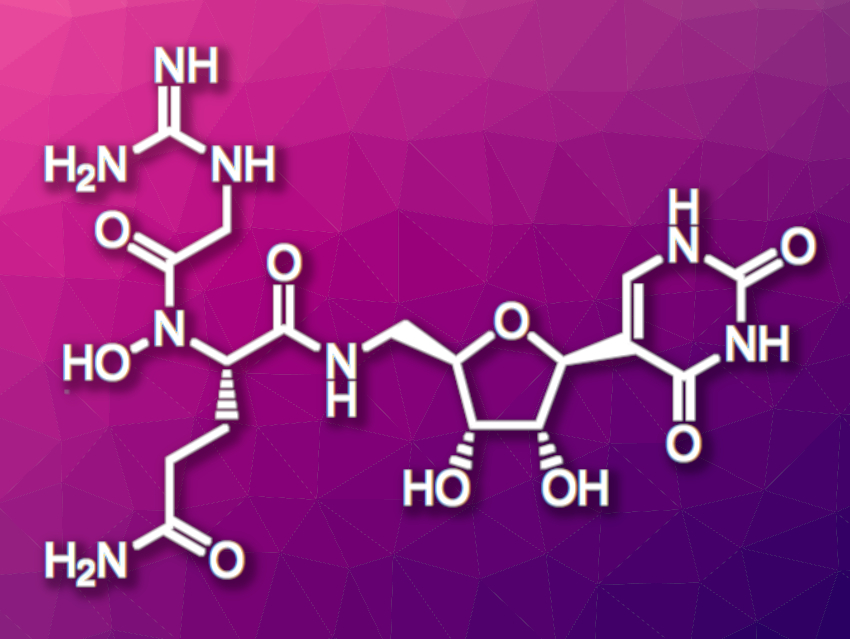
Pseudouridimycin (pictured) is a natural product with antimicrobial activity. It inhibits bacterial RNA polymerase, which catalyzes the transcription of DNA into RNA and, thus, is essential to gene expression. This makes bacterial RNA polymerase a possible target for antimicrobial drugs.
Juan R. Del Valle, University of Notre Dame, IN, USA, and colleagues have performed a total synthesis of pseudouridimycin, as well as three new variants of the compound. This work could help with the development of pseudouridimycin analogues with improved stability and/or activity. The team first synthesized a protected β-pseudouridine derivative, starting from a D-ribonolactone derivative. This compound was reacted with lithiated 2,4-di-t-butylpyrimidine, followed by a reduction, a cyclization, and an azidation.
The researchers prepared the dipeptide unit of pseudouridimycin starting from a commercially available glutamine derivative, which was converted to a hydroxylamine and acylated. Condensation with a primary amine derived from the azide-functionalized β-pseudouridine unit, followed by deprotection, then gave the desired pseudouridimycin. The product was obtained in 21 % overall yield in ten steps (longest linear sequence). Following similar synthetic pathways, the team prepared three different pseudouridimycin analogues that differ from the parent compound in the dipeptide unit. This could help to optimize the properties of pseudouridimycin for pharmaceutical use.
- Total synthesis and chemical stability of pseudouridimycin,
Juan Del Valle, Christopher F. Cain, Aaron M. Scott, Matthew P. Sarnowski,
Chem. Commun. 2022.
https://doi.org/10.1039/d1cc07059b