Coronene is a polycyclic aromatic hydrocarbon composed of seven fused benzene rings. Such π-conjugated systems can have useful optoelectronic properties. Replacing pairs of two adjacent carbon atoms with B–N units could be useful to tune those properties for specific applications. However, the selective synthesis of polycyclic aromatic hydrocarbons with B–N units in specific positions can be challenging.
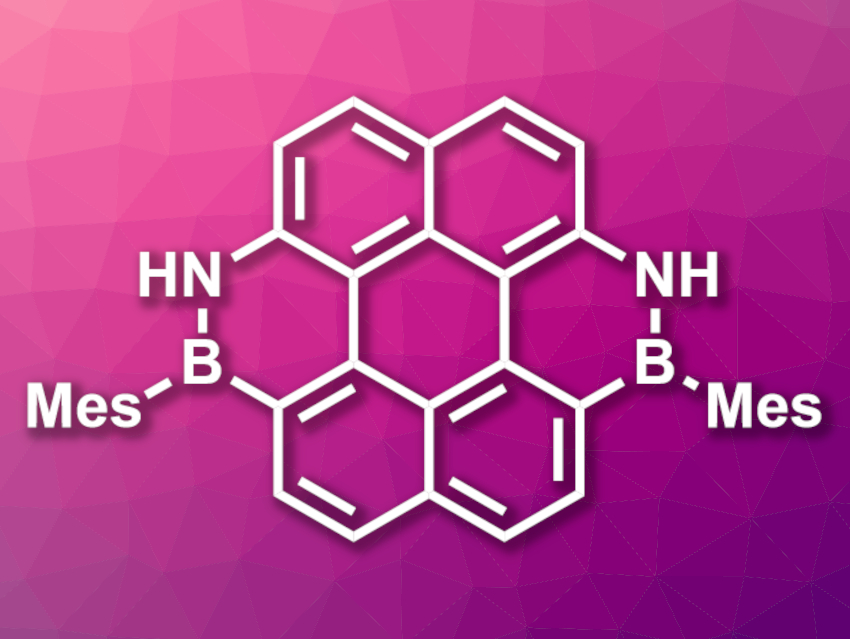
Zhiqiang Liu, Shandong University, Jinan, and Shenzhen Research Institute of Shandong University, both China, Xiaoqiang Yu, Shandong University, and colleagues have synthesized BN-embedded benzo[ghi]perylene (Bzp) and coronene (Cor) derivatives (latter pictured, Mes = mesityl). The team used binaphthyl precursors functionalized with amino groups, which were reacted with BBr3 and quenched with mesityl magnesium bromide. The Bzp- and Cor-based products were obtained in yields of 38 % and 35 %, respectively.
The team evaluated the photophysical properties of the products using UV–Vis absorption and fluorescence emission spectroscopy and compared them with the corresponding all-carbon analogues. They found that the BN-embedded variants have larger absorption coefficients and much higher fluorescence quantum yields than their analogues without BN units. According to the researchers, the strategy could also be used to construct other, more complex BN-containing polycyclic aromatic hydrocarbons.
- Synthesis, Structure, and Photophysical Properties of BN-Embedded Analogue of Coronene,
Zhen Jiang, Shimin Zhou, Wendong Jin, Cuihua Zhao, Zhiqiang Liu, Xiaoqiang Yu,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.1c04161