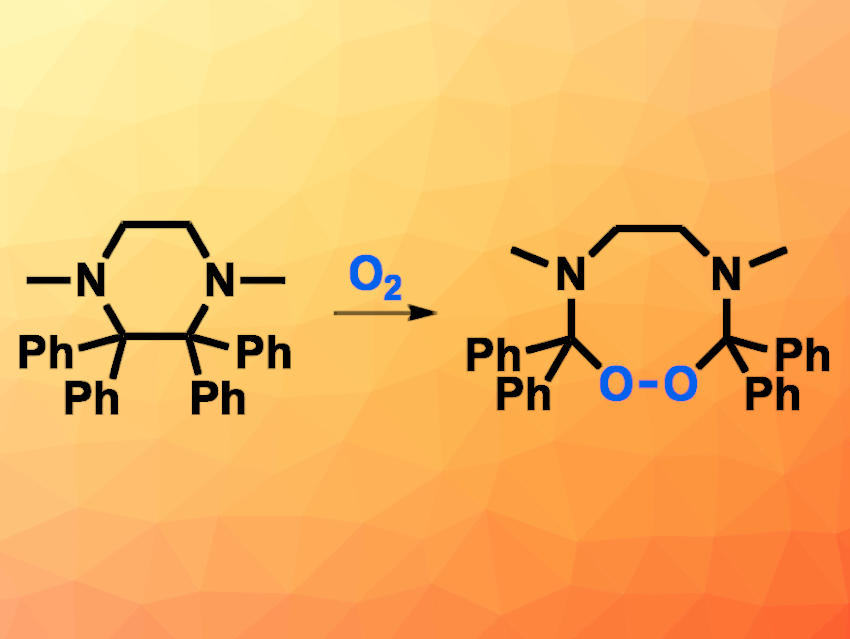
The activation of molecular oxygen (O2) can be useful, e.g., for the synthesis of organic peroxides. O2 can react with organic radicals or unsaturated compounds, for example. It can also react with organic compounds across a C(sp3)–C(sp3) bond in the presence of transition-metal catalysts.
Swapan K. Pati, Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, India, Prasanta Ghosh, Ramakrishna Mission Residential College, Kolkata, India, Carola Schulzke, University of Greifswald, Germany, Anukul Jana, Tata Institute of Fundamental Research Hyderabad, India, and colleagues have found a previously unknown activation of O2 across a C(sp3)–C(sp3) bond without any catalyst in a piperazine derivative (pictured).
The team synthesized the piperazine derivative starting from benzophenone and ethylenediamine, which were converted to an N,N’-ethylene-bridged bis-ketinium cation. A reaction with KC8 then gave the desired substituted piperazine.
The piperazine reacted with O2 from air in a hexane solution to give the corresponding 1,2,4,7-dioxadiazoctane derivative, an eight-membered ring that contains a peroxide group. The team found that the substitution of the piperazine is important for the reaction: A derivative with single instead of double phenyl substitution at the neighboring C atoms did not react with O2.
- Activation of O2 across a C(sp3)–C(sp3) bond,
Rahul Kumar, Stefan Richter, Suvendu Maity, Pallavi Sarkar, Nicolas Chrysochos, Swapan K. Pati, Prasanta Ghosh, Carola Schulzke, Anukul Jana,
Chem. Commun. 2022.
https://doi.org/10.1039/d2cc00110a