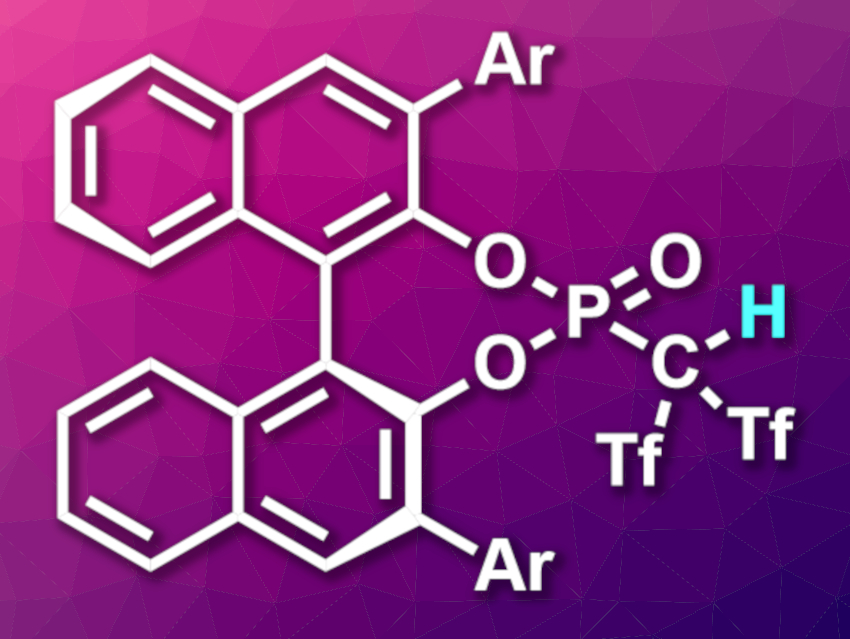
Brønsted acid catalysis is a useful tool in organic synthesis. Chiral Brønsted acid catalysts, such as 1,1′-bi-2-naphthol (BINOL)-derived chiral phosphoric acids or N-triflyl phosphoramides, for example, are particularly interesting in this context. Generally, higher acidity leads to higher reactivity, but very strong chiral Brønsted acids are rare.
Wen-Wen Chen, Baoguo Zhao, Shanghai Normal University, China, Kuiling Ding, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and colleagues have developed a new type of chiral super Brønsted C–H acids, BINOL-derived phosphoryl bis((trifluoromethyl)sulfonyl) methanes (BPTMs, pictured). The trifluoromethanesulfonyl, or triflyl (Tf), groups have strong electron-withdrawing properties, and thus, raise the acidity of the compounds. The team synthesized the acids from a 3,3′-diiodide-substituted phosphoryl chloride derivative, which was reacted with an in–situ-generated bis((trifluoromethyl)sulfonyl)methyl dianion. The aryl substituents in the 3,3′-positions were then introduced via Suzuki coupling reactions.
The prepared BPTMs were used as chiral Brønsted acid catalysts in asymmetric Mukaiyama–Mannich reactions, asymmetric allylic aminations, coupling reactions of allyltrimethylsilane with 9-fluorenylmethyl carbamate and aldehydes, and the protonation of silyl enol ethers. The BPTM catalysts showed good to excellent enantioselectivities and improved activities compared with BINOL-derived chiral phosphoric acids and N-triflyl phosphoramides.
- A Powerful Chiral Super Brønsted C–H Acid for Asymmetric Catalysis,
Bingfei Peng, Jiguo Ma, Jianhua Guo, Yating Gong, Ronghao Wang, Yi Zhang, Jinlong Zeng, Wen-Wen Chen, Kuiling Ding, Baoguo Zhao,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.1c12723