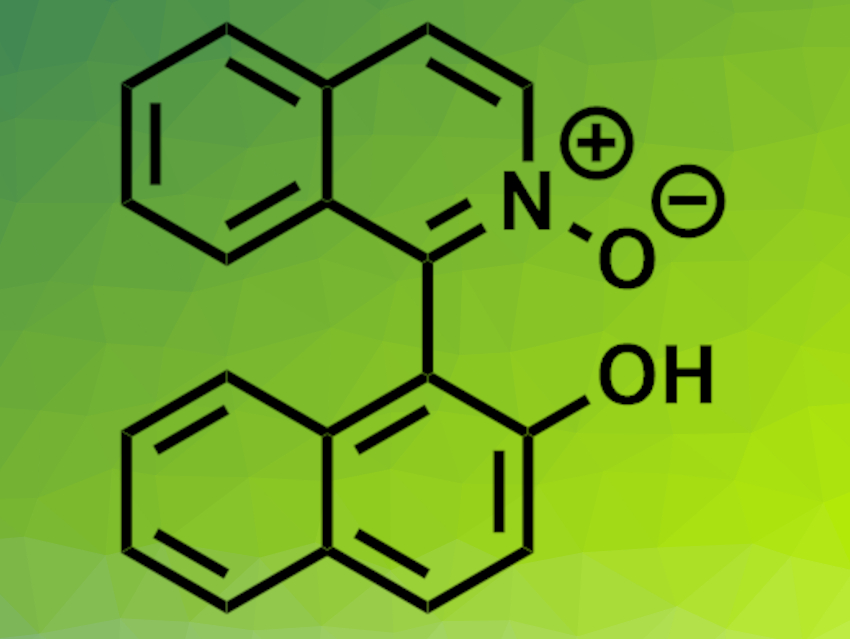
Axially chiral biaryls are useful ligands. Heterobiaryl ligands have two different donor atoms, which is interesting for catalytic applications. Axially chiral isoquinolinyl-naphtholate ligands, for example, have N- and O binding sites. Derivatives containing quinoline-type N-oxides (example pictured) can act as organocatalysts. Despite their useful properties, there is a lack of general methods for the synthesis of heterobiaryls.
Rajarshi Samanta, Indian Institute of Technology Kharagpur, India, and colleagues have developed a method for the Cu(II)-catalyzed synthesis of heterobiaryls using easily available N-oxides and diazonaphthoquinones. The team reacted different isoquinoline or phthalazine N-oxide derivatives with diazonaphthoquinones, using copper(II) triflate (Cu(OTf)2) as a catalyst, 2,2′-bipyridine as a ligand, and a mixture of dichloromethane (DCM) and PhCF3 as the solvent.
The desired products were obtained in moderate to good yields. The researchers propose a reaction mechanism that involves a deprotometalation with the active copper catalyst at the N-oxide component, followed by the formation of a metal-carbene-intermediate with the diazonaphthoquinone substrate under loss of N2. A migratory insertion and a protodemetalation then give the coupling product. The heterobiaryl products can be further transformed to obtain, e.g., an OMe-functionalized ligand or structurally related P,N ligands.
- Cu(II)-Catalyzed Construction of Heterobiaryls using 1-Diazonaphthoquinones: A General Strategy for the Synthesis of QUINOX and Related P,N Ligands,
Aniruddha Biswas, Subarna Pan, Rajarshi Samanta,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00127