Cyclopentadienyl (Cp) ligands are widely used in organometallic chemistry. One well-known example of a Cp complex is the highly stable iron sandwich complex ferrocene. Many other metals also form cyclopentadienyl complexes, but depending on the coordinated metal and its oxidation state, these complexes are often much less stable than ferrocene. Such complexes can generally be stabilized by the addition of bulky substituents at the Cp ring that sterically shield the metal center.
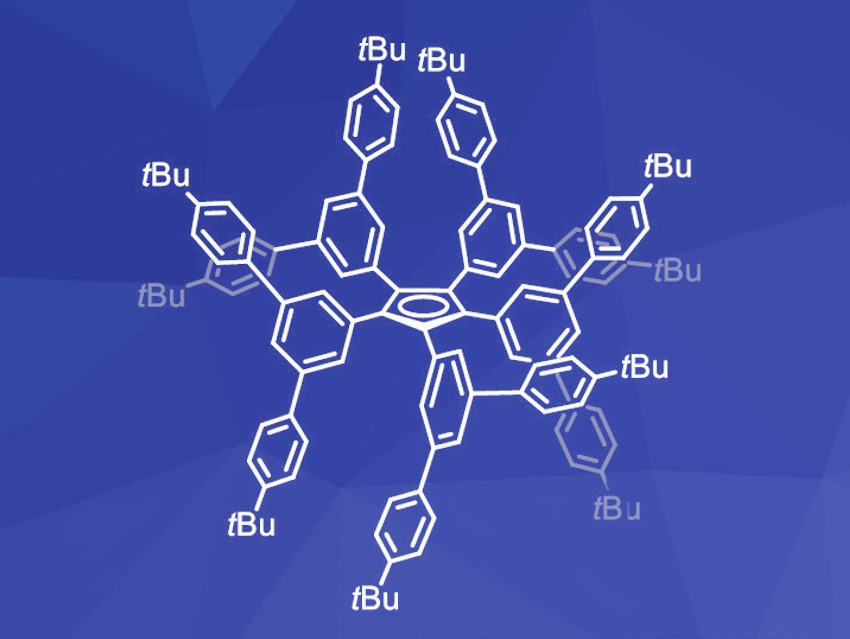
Robert Wolf and Gabriele Hierlmeier, University of Regensburg, Germany, have developed an extremely bulky cyclopentadienyl ligand in which every carbon atom of the Cp ring is linked to a terphenyl unit, which is further substituted by tert-butyl groups (CpT5, pictured). The ligand was synthesized via a Pd-catalyzed Heck coupling reaction of cyclopentadiene and the respective terphenyl bromide. After 72 h at 130 °C in dimethylformamide (DMF), the protonated ligand was obtained in 89 % yield and characterized by NMR spectroscopy, mass spectrometry (MS), and X-ray diffraction.
The synthesized ligand was reacted with various alkali metals and alkali metal bases. A reaction with n-BuLi, for example, proceeded very slowly. After four weeks at room temperature, a green solid was formed. This product was the desired lithium salt, containing a lithium–CpT5 sandwich structure. CpT5 did not react with sodium metal, but when sodium hexamethyldisilazide (NaHMDS) was used, the sodium salt was formed overnight at 80 °C. The structure of the sodium–CpT5 complex is analogous to the Li compound. Potassium metal in hexane reacts directly with CpT5, forming the first potassocene complex. The solid-state structure of potassium–CpT5 differs from the lighter analogues: While one potassium cation is sandwiched between two Cp rings, the second one is coordinated by the aryl rings of two terphenyl substituents. The corresponding rubidium and cesium salts were also synthesized successfully.
These results show that the steric bulk of CpT5 enables the formation of unusual alkali-metal coordination compounds. This principle may be extended to other complexes in future studies.
- Bulking up CpBIG: A Penta-Terphenyl Cyclopentadienyl Ligand,
Gabriele Hierlmeier, Robert Wolf,
Organometallics 2022.
https://doi.org/10.1021/acs.organomet.2c00009