Elemental phosphorus usually forms structures with single bonds, unlike the lighter, triple-bonded N2. Due to its electron configuration, the reactivity of the diphosphorus molecule P2 might have more in common with that of acetylene (HC≡CH) than with that of N2. However, the triple bond in P2 is significantly weaker than in acetylene, and it is, thus, highly reactive and unstable. Coordination might be used to stabilize P2, but coordination compounds that contain P2 bound side-on to a single metal center, analogous to an acetylene ligand, had remained unknown so far.
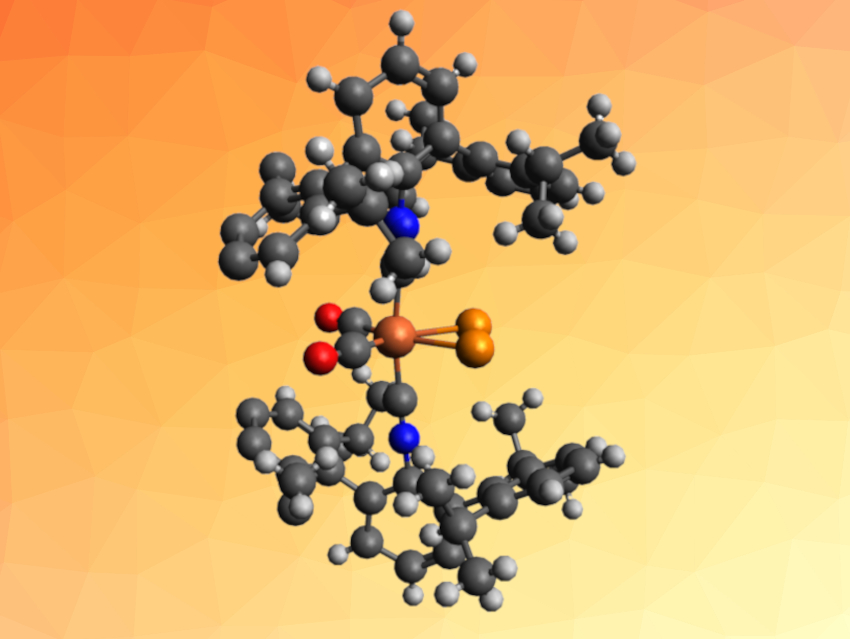
Joshua S. Figueroa, University of California, San Diego, La Jolla, USA, and colleagues have synthesized a mononuclear iron complex that contains a P2 molecule coordinated in a side-on, η2-binding mode (pictured). To obtain the P2 complex, the team reacted the complex K2[Fe(CO)2(CNArDipp2)2] (ArDipp2 = 2,6-(2,6(iPr)2C6H3)2C6H3) with two equivalents of the anthracendiyl-substituted chlorophosphine ClP(anth). In this approach, ClP(anth) serves as a phosphorus source for the formation of diphosphorus.
The desired mononuclear, side-on diphosphorus complex (η2-P2)Fe(CO)2(CNArDipp2)2 (pictured) was obtained in the form of a yellow solid. The product was characterized using X-ray crystallography and NMR spectroscopy, and its bonding situation was studied using density functional theory (DFT) calculations. The P2 complex was compared to an analogous η2-bound bis-trimethylsilylacetylene iron complex.
The team found that the P2 complex is isostructural to the acetylene complex in the solid state. However, the reactivities of the two ligands are expected to differ significantly. The researchers anticipate that the binding mode of the P2 ligand might enable selective phosphorus-atom transfer reactions to organic molecules.
- Side-on coordination of diphosphorus to a mononuclear iron center,
Shuai Wang, Jeffrey D. Sears, Curtis E. Moore, Arnold L. Rheingold, Michael L. Niedig, Joshua S. Figueroa,
Science 2022.
https://doi.org/10.1126/science.abn7100