Ferrocene is a commonly used organometallic compound in which two cyclopentadienyl ligands form a “sandwich” around an iron center. Inorganic analogues of ferrocene might be created, e.g., by exchanging all C–H groups in the cyclopentadienyl ligands with P atoms. However, all-phosphorus sandwich complexes are rare, and the closest heteroelement analogue of ferrocene until now had been the complex [Ti(η5-P5)2]2–. The all-phosphorus analogue of ferrocene, Fe(η5-P5)2, has not been synthesized so far.
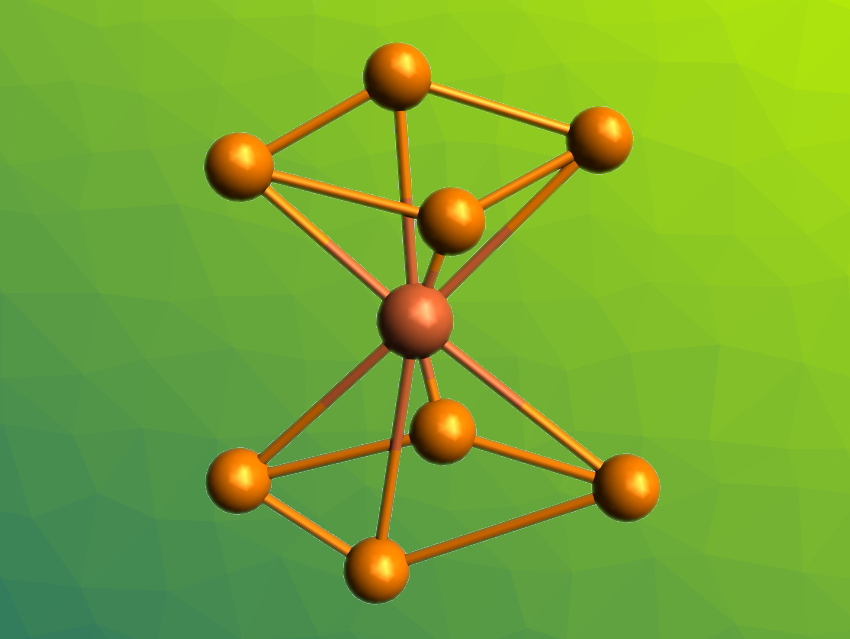
Zhong-Ming Sun, Nankai University, Tianjin, China, and colleagues have prepared the iron complex [Fe(P4)2]2– (pictured), which is the first homoleptic iron complex containing only cyclo-Pn ligands and can be considered the closest iron-containing analogue of ferrocene with all-phosphorus ligands so far. The team used the Zintl phase KP as a precursor, dissolved it in ethylenediamine (en) in the presence of the cryptand 2,2,2-crypt, and reacted it with FeOtBu3. They obtained crystals of [K(2,2,2-crypt)]2[Fe(P4)2] and [K(2,2,2-crypt)]3[(P7)Fe(P4)].
Based on X-ray diffraction, the team found that [K(2,2,2-crypt)]2[Fe(P4)2] crystallizes in triclinic space group P1. They observed complete molecular ion peaks in the results of electrospray-ionization mass spectrometry (ESI-MS) analyses, which indicates a high stability of the molecular anion. [Fe(P4)2]2– has a sandwich-type structure with the two ligands in a staggered conformation. According to the researchers, the work demonstrates the potential of Zintl-type precursors for the preparation of ionic analogues of homoleptic all-phosphorus metallocene derivatives.
- Inorganic Ferrocene Analogue [Fe(P4)2]2–,
Zi-Chuan Wang, Lei Qiao, Zhong-Ming Sun, Manfred Scheer,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c01750