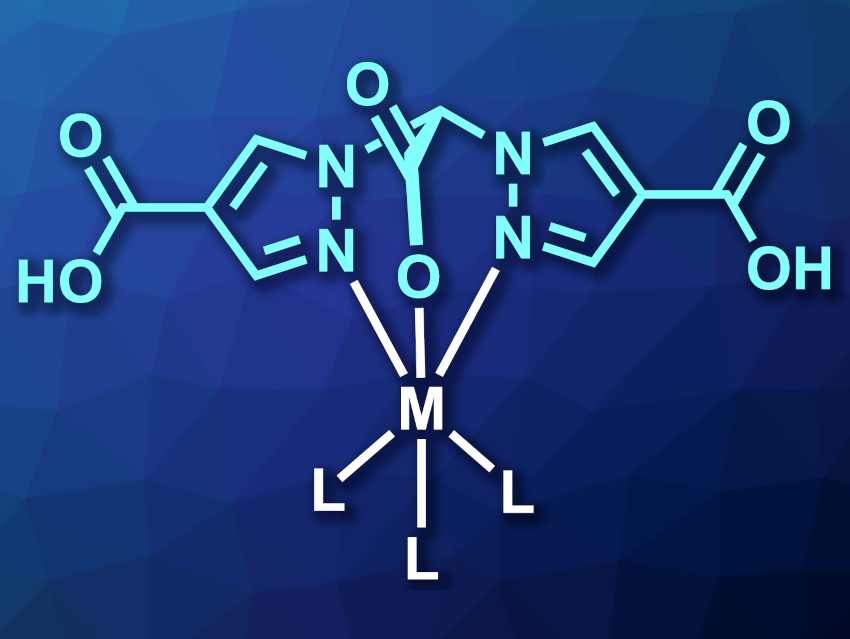
Scorpionate ligands have three binding sites, similar to the two pincers plus the stinger of a scorpion. Bis(pyrazol-1-yl)acetic acids, for example, are heteroscorpionate ligands with two nitrogen binding sites and one oxygen binding site. They are widely used ligands. However, improving the water solubility of the resulting complexes, e.g., for biological applications, is still an open challenge.
Nicolai Burzlaff, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, and colleagues have prepared the heteroscorpionate ligand bis(4-carboxylpyrazol-1-yl)acetic acid (H3bcpza) (H2bcpza– pictured in cyan) in a one-pot process. The two additional peripheral carboxyl groups (compared with bis(pyrazol-1-yl)acetic acid) improve the solubility of the resulting complexes (general structure pictured) in aqueous solutions.
The team prepared the ligand using a phase-transfer-catalyzed procedure, starting from the commercially available ester methyl pyrazole-4-carboxylate. This ester was reacted with dibromoacetic acid in the presence of KOH/K2CO3 as bases and benzyltriethylammonium chloride as a phase-transfer catalyst. The desired product was obtained in a yield of 74 %.
The researchers then synthesized the complexes [Mn(H2bcpza)(CO)3], [Re(H2bcpza)(CO)3], and [Ru(H2bcpza)Cl(CO)2]. They found that the complexes are partially soluble in water and readily soluble in phosphate-buffered saline (PBS) buffer solution due to deprotonation of the additional carboxylic acid groups.
- Bis(4-carboxylpyrazol-1-yl)acetic acid: A scorpionate ligand for complexes with improved water solubility,
Wintana Tzegai, Michaela Reil, Nicolai Burzlaff,
Dalton Trans. 2022.
https://doi.org/10.1039/d2dt00848c