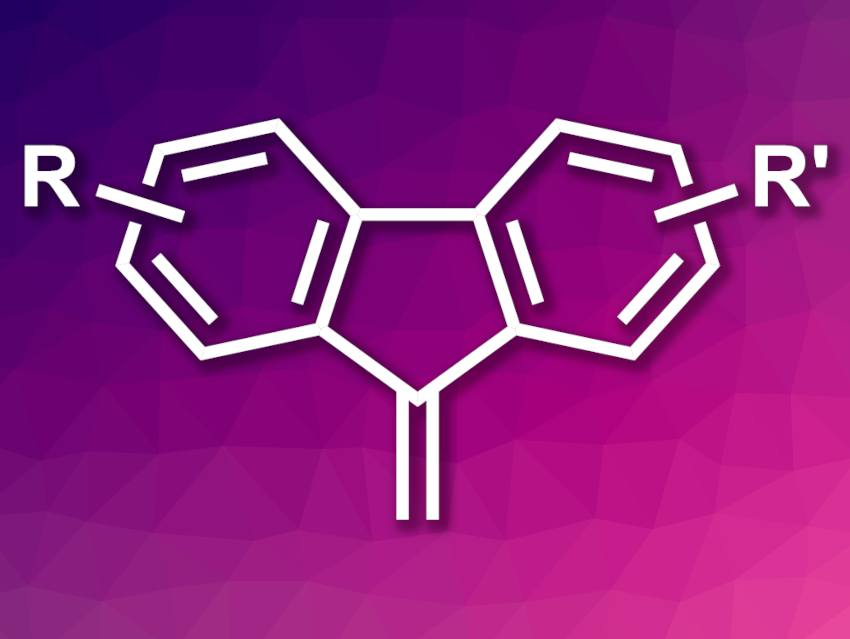
Cycloisomerization reactions of 2-alkynylbiaryls provide an atom-economic approach to the synthesis of fused-ring systems. They usually give phenanthrene derivatives, while 9-alkylidene fluorene derivatives are rarely produced.
Sheng Zhang, Ming Bao, Dalian University of Technology, China, and colleagues have developed a palladium-catalyzed cycloisomerization of 2-ethynylbiaryls to 9-methylidene fluorenes (general structure pictured). Due to the reactive exocyclic C=C bond, 9-methylidene fluorenes are useful products that can easily be further transformed and functionalized. The team used Pd2(dba)3, i.e., (tris(dibenzylideneacetone)dipalladium(0), as a pre-catalyst, t-BuPPh2 as a ligand, adamantane carboxylic acid as an additive, and tetrahydrofuran (THF) as the solvent. The reactions were performed at 35 °C.
Under these conditions, the researchers converted a wide variety of 2-ethynylbiaryls to the corresponding 9-methylidene fluorene derivatives. The desired products were obtained in moderate to good yields. The team proposes a reaction mechanism that involves a hydropalladation followed by aromatic C–H activation and reductive elimination.
- Palladium-Catalyzed Cycloisomerization of 2-Ethynylbiaryls to 9-Methylidene Fluorenes,
Hongyu Guo, Sheng Zhang, Xiujuan Feng, Xiaoqiang Yu, Yoshinori Yamamoto, Ming Bao,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00534