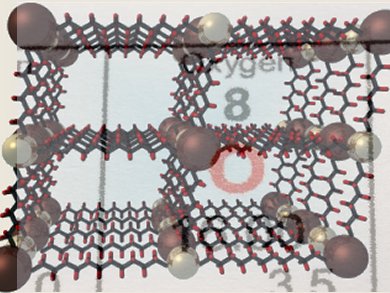
With over 100 million tons produced annually, O2 is one of the most widely used commodity chemicals in the world. The separation of O2 from air is carried out on a large scale using an energy-intensive cryogenic distillation process.
Jeffrey Long and colleagues, University of California, Berkeley, USA, have synthesized a new microporous metal–organic framework (MOF) with open iron(II) coordination sites. The MOF selectively binds O2 over N2. At 211 K, O2 uptake is fully reversible with a capacity of 18.2 wt %, corresponding to the adsorption of one O2 molecule per iron center. Ideal adsorbed solution theory indicates that the material should be capable of high-capacity separation at temperatures as high as 226 K.
Being able to separate O2 from air at this temperature, which is substantially higher than the temperatures currently used, would lower energy and capital costs.
- Selective Binding of O2 over N2 in a Redox–Active Metal–Organic Framework with Open Iron(II) Coordination Sites
E. D. Bloch, L. J. Murray, W. L. Queen, S. Chavan, S. N. Maximoff, J. P. Bigi, R. Krishna, V. K. Peterson, F. Grandjean, G. J. Long, B. Smit, S. Bordiga, C. M. Brown, J. R. Long,
J. Am. Chem. Soc. 2011.
DOI: 10.1021/ja205976v