Molecules bearing trifluoromethyl groups have been widely applied in the fields of functional materials, agrochemicals, and pharmaceuticals due to their hydrophobicity, metabolic stability, and bioavailability.
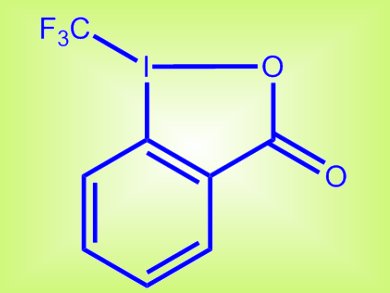
Jianbo Wang and colleagues, Peking University, China, report a route to synthesize allylic trifluoromethylated compounds under mild conditions. The reaction employs cheap copper chloride as the catalyst and a hypervalent iodine(III) reagent (pictured) as both the oxidant and the CF3 source. The hypervalent iodide reagent is easily accessible from commercially available 2-iodobenzoic acid and provided the desired products in up to 97 % isolated yield. Simple alkenes can be used as substrates and the reaction tolerates a wide range of functional groups.
This methodology represents a rare example of a general and highly efficient direct trifluoromethylation of terminal olefins.
- Copper-Catalyzed C(sp3)–C(sp3) Bond Formation Using a Hypervalent Iodine Reagent: An Efficient Allylic Trifluoromethylation
X. Wang, Y. Ye, S. Zhang, J. Feng, Y. Xu, Y. Zhang, J. Wang,
J. Am. Chem. Soc. 2011.
DOI: 10.1021/ja207775a