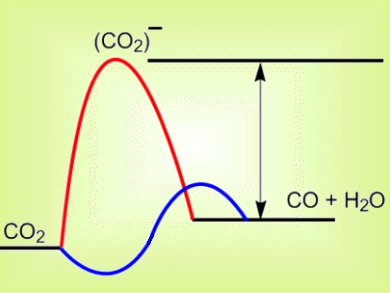
The first step in CO2 conversion in electrochemical cells for production of renewable sources of formic acid or methanol is the formation of a (CO2)− intermediate. The equilibrium potential for (CO2)− formation is very negative so for the reaction to occur, it is necessary to run the cathode very negative, i.e., at a high overpotential (pictured, arrow). A few homogeneous catalysts show initial activity at relatively low overpotentials of 0.6 V, but most quickly lose their activity.
Richard Masel and colleagues, Dioxide Materials, Illinois, USA, have significantly reduced the overpotential required for CO2 conversion. They add an ionic liquid electrolyte to the cell to lower the energy of the (CO2)– intermediate (pictured, blue). The ionic liquid most likely formed a complex with the intermediate, and thereby lowered the initial reduction barrier. This allows CO to be produced at an applied voltage of 1.5 V, only 0.2 V above the equilibrium voltage. The cell was be run for over seven hours with no significant loss of activity.
- Ionic Liquid–Mediated Selective Conversion of CO2 to CO at Low Overpotentials
B. A. Rosen, A. Salehi-Khojin, M. R. Thorson, W. Zhu, D. T. Whipple, P. J. A. Kenis, R. I. Masel,
Science 2011.
DOI: 10.1126/science.1209786