Sterically hindered or frustrated Lewis acid–Lewis base pairs (FLPs) are of interest because of their ability to activate small molecules, including CO2 and H2. Typical examples are inter- or intramolecular combinations of bulky phosphines or amines with strongly electrophilic RB(C6F5)2 components.
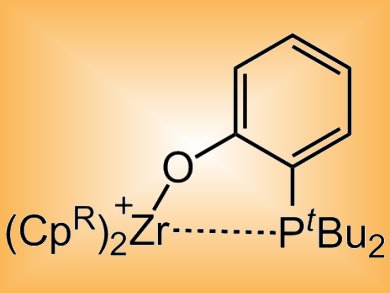
Duncan Wass and co-workers, University of Bristol, UK, have developed transition metal-based analogues of FLPs that undergo additional reactivity. The cationic zirconocene–phosphinoaryloxide complexes (pictured) mirror the reactivity of main group systems for the heterolytic cleavage of H2, activation of CO2, alkenes, alkynes and aldehydes, and the ring-opening of THF. The complexes were also shown to go beyond the main group systems reported to date in accessing more powerful reactivity. This includes the stepwise reduction of CO or CO2 under mild conditions, the cleavage of C–O bonds in acyclic ethers, and the cleavage of aliphatic C–Cl or even C–F bonds.
- Frustrated Lewis Pairs beyond the Main Group: Synthesis, Reactivity, and Small Molecule Activation with Cationic Zirconocene–Phosphinoaryloxide Complexes
A. M. Chapman, M. F. Haddow, D. F. Wass,
J. Am. Chem. Soc. 2011.
DOI: 10.1021/ja207936p
See also:
- Frustrated Lewis Pairs: Metal-free Hydrogen Activation and More
D. W. Stephan, G. Erker,
Angew. Chem. Int. Ed. 2010, 49(1), 46–76.
DOI: 10.1002/anie.200903708